What should be done before implementing CAR-T therapy?
Precautions before CAR-T therapy
Patient's condition should be assessed before receiving CAR-T therapy
Patients who are to be treated with commercial CAR-T cell products need to be assessed by a physician for their suitability to receive CAR-T cell therapy.
Advantages of commercial CAR-T cell products
Safety: Fully enclosed operation, strictly following GMP Class A standard operating environment
Efficacy: Clear efficacy, test and release specifications reviewed and approved by CDE
Quality control: Commercially mature preparation platform, whole-process monitoring
China Medicinal Biotechnology Association. Code of Manufacturing Quality Management for Chimeric Antigen Receptor T Cells (CAR-T cells) Based-Medicinal Product
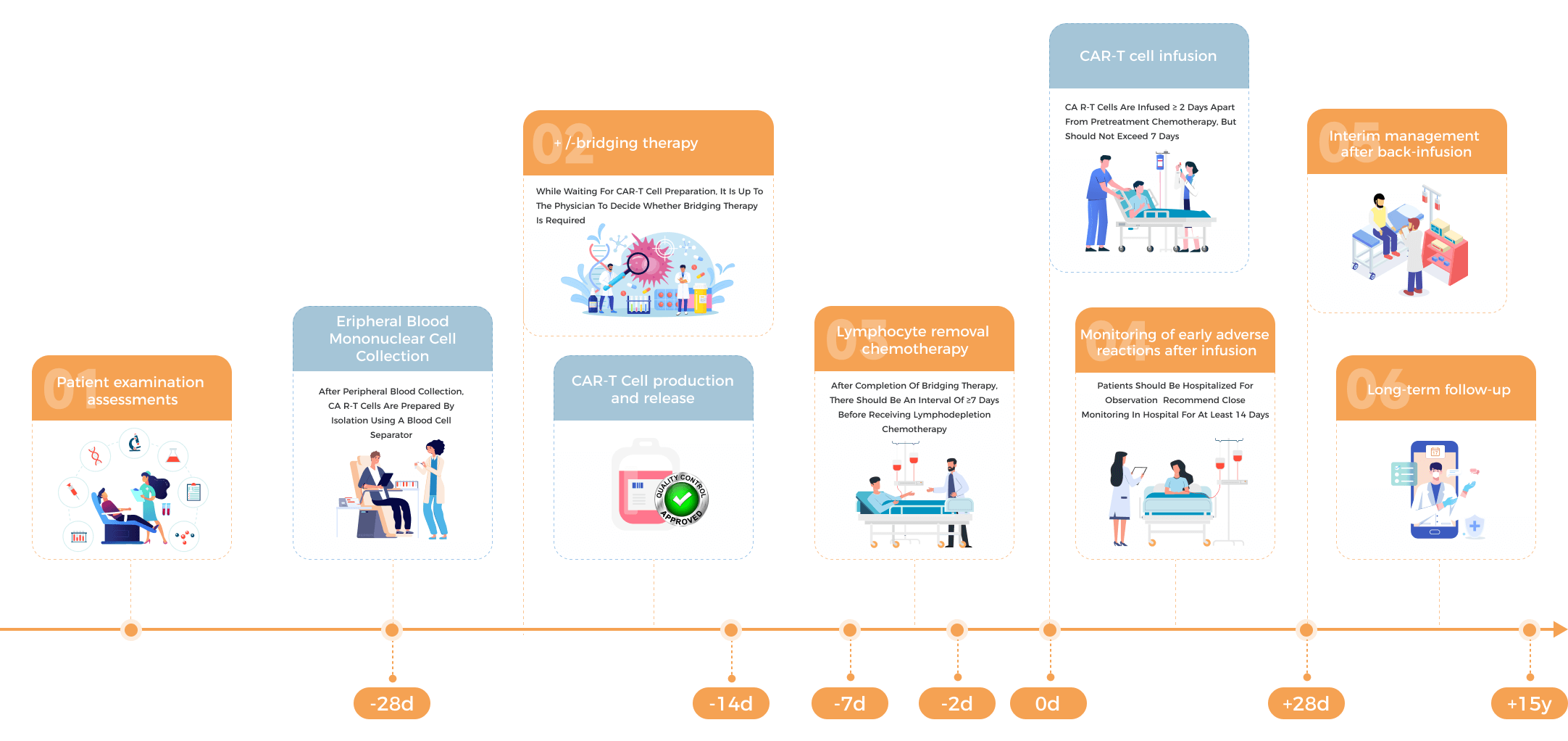
What is the procedure of CAR-T therapy for myelomas?
Flow Chart for Myeloma Patients Receiving CAR-T Therapy
The unique feature of CAR-T therapy
1.Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Management of Infections Associated with CAR-T Cells in the Treatment of Hematologic Malignancies and Immune Targeted Therapy 2022 [M]. Beijing: People's Medical Publishing House, 2022.
2.Hematology Branch of Chinese Medical Doctor Association, Hematology Branch of Chinese Medical Association. Chinese Journal of Hematology, 2022, 43 (04): 265-271.
What assessment should be performed prior to peripheral blood mononuclear cells collection?

General condition assessment
- Symptoms
- Vital signs
- Performance status assessment (ECOG score)

Laboratory tests
- Blood/urine/stool routine tests
- Blood chemistry panel
- Coagulation function
- Infection screening (hepatitis B, hepatitis C, syphilis, HIV)
- HBV DNA

Prior therapy
- A washout period is required for prior medications
Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Management of Infections Associated with CAR-T Cells in the Treatment of Hematologic Malignancies and Immune Targeted Therapy 2022 [M]. Beijing: People's Medical Publishing House, 2022.
What should be noted during peripheral blood mononuclear cell collection?
The following should be considered during peripheral blood mononuclear cell collection:
Effect of prior therapeutic agents
Lymphotoxic drugs, such as fludarabine, cladribine, lenalidomide, and glucocorticoids, should have a washout period.
01
Pay attention to the number of lymphocytes
It is generally recommended that the absolute lymphocyte count before apheresis be ≥ 0.1 × 109 L, and if it is > 0.5 × 109 L, the likelihood of successful expansion in vivo after infusion of CAR-T cells is higher.
02
Lymphocytes should be collected in advance for backup
Patients at high risk or requiring lenalidomide may be considered to avoid the effect of prior lines of therapy and some drugs on CAR-T cell activity.
03
1.Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Management of Infections Associated with CAR-T Cells in the Treatment of Hematologic Malignancies and Immune Targeted Therapy 2022 [M]. Beijing: People's Medical Publishing House, 2022.
2.Hematology Branch of Chinese Medical Doctor Association, Hematology Branch of Chinese Medical Association. Chinese Journal of Hematology, 2022, 43 (04): 265-271.
Prevention and management of adverse reactions during peripheral blood mononuclear cell collection
Most complications during the apheresis are mild and controllable, such as paresthesia, pain, nausea, vomiting, and headache.
Hypocalcemia:It is the most common adverse reaction. Elderly women and frail patients can take oral calcium supplements for prevention, or improve symptoms by intravenous drip or slow intravenous bolus injection of calcium gluconate during the separation process.
Hypovolemic syndrome: This is relatively rare. Patients should avoid collection on an empty stomach and pay attention to their own condition during the collection process. If there is any discomfort, please report to the doctor in time.
Zhitao Ying, Ningjing Lin, Meng Wu, et al. Leukemia · Lymphoma, 2021, 30 (11): 674-684.
How to determine the need of bridging therapy?
Prior to initiating CAR-T cell therapy, assessing the potential requirement for bridging therapy is of paramount importance. Considerations for this interim treatment encompass the aggressiveness of the disease, potential delays in CAR-T cell production, and the overall health status of the patient. If there is a heightened risk of rapid disease progression, bridging therapy may be administered to mitigate tumor burden, alleviate associated symptoms, and ensure a smooth transition to subsequent treatment. Consultation with the treatment team is crucial before implementing bridging therapy. Thorough discussions should cover the necessity, specific objectives, impact on the overall treatment plan, and potential alternative bridging therapies. Typically, physicians choose previously effective drugs based on the patient's response to prior treatments, opting for previously used medications or exploring unused yet potentially effective options, such as chemotherapy, targeted therapy, immunotherapy, and radiation. This ensures a well-informed decision-making process addressing immediate disease management needs before embarking on CAR-T cell therapy.
Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Management of Infections Associated with CAR-T Cells in the Treatment of Hematologic Malignancies and Immune Targeted Therapy 2022 [M]. Beijing: People's Medical Publishing House, 2022.
Why should lymphodepletion chemotherapy be performed before infusion?
Since CAR-T cells are considered "strangers" to the human immune system, direct infusion may lead to the clearance of CAR-T cells by the immune system (mainly lymphocytes) and failure in the treatment of diseases.
Therefore, lymphodepletion chemotherapy should be performed before infusion to clear normal lymphocytes in the patient's body and create an environment conducive to the survival and growth of CAR-T cells, which can also upregulate tumor immunogenicity and improve disease control.
1.Zhitao Ying, Ningjing Lin, Meng Wu, et al. Leukemia · Lymphoma, 2021, 30 (11): 674-684.
2.Yakoub-Agha I, et al. Haematologica. 2020 Jan 31;105(2):297-316.
What tests are required before lymphodepletion chemotherapy?
Prior to lymphodepleting chemotherapy, a comprehensive assessment is imperative to ensure the patient's ability to safely tolerate the treatment. First and foremost, confirmation of the successful preparation of the CAR-T cell product is essential. Laboratory examinations encompass a complete blood count, blood smear, urinalysis, comprehensive biochemical profile, and coagulation function tests, facilitating an evaluation of the patient's baseline physiological status. Additionally, essential imaging studies such as an electrocardiogram (ECG) are conducted. For patients undergoing bridging therapy or with extramedullary lesions, a localized CT/MRI examination is essential to comprehensively understand the lesion's status before treatment. Physical examination extends beyond an overall health evaluation, encompassing an assessment of vital signs, ECOG performance status (reflecting the patient's daily living activities), and the Immune Cell-Related Encephalopathy (ICE) score. This thorough and detailed examination protocol aims to ensure that patients are in optimal condition before undergoing lymphodepleting chemotherapy, thereby minimizing treatment risks.
Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Management of Infections Associated with CAR-T Cells in the Treatment of Hematologic Malignancies and Immune Targeted Therapy 2022 [M]. Beijing: People's Medical Publishing House, 2022.
What should be noted for CAR-T cell infusion?

Before infusion, it is necessary to confirm that the prepared CAR-T cell product matches the patient's identification and to verify the patient's condition, including general condition assessment and laboratory tests

Acetaminophen derivatives and antihistamines (such as chlorpheniramine or diphenhydramine) will be used for pretreatment 30-60 minutes before infusion

Before and after the infusion, the clinician will ensure that there are enough tocilizumab and corresponding emergency equipment available

During and after the infusion, the granulocyte-macrophage colony-stimulating factor (GM-CSF) is prohibited
Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Management of Infections Associated with CAR-T Cells in the Treatment of Hematologic Malignancies and Immune Targeted Therapy 2022 [M]. Beijing: People's Medical Publishing House, 2022.
How should the patient be monitored after infusion?
Following CAR-T therapy, a meticulous two-week monitoring period is indispensable. This surveillance should encompass patient status assessments, monitoring of CAR-T cell proliferation and adverse reactions, and an evaluation of CAR-T treatment efficacy. Patient status assessments involve routine examinations and laboratory tests to gain a comprehensive understanding of the patient's overall health. Additionally, close monitoring of CAR-T cell proliferation within the patient's body is imperative to ensure treatment effectiveness. Early detection and monitoring of adverse reactions are pivotal for prompt intervention. Finally, utilizing imaging studies and bone marrow examinations aids in assessing the efficacy of CAR-T treatment, providing insights into post-treatment tumor status and the patient's overall response. This comprehensive and systematic monitoring protocol enhances the success rate of CAR-T treatment while minimizing potential treatment risks.
Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Management of Infections Associated with CAR-T Cells in the Treatment of Hematologic Malignancies and Immune Targeted Therapy 2022 [M]. Beijing: People's Medical Publishing House, 2022.
Adverse Reactions and Management After CAR-T Cell Infusion
Following CAR-T cell therapy, patients may experience varying degrees of adverse reactions, including Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). CRS symptoms may range from fever and fatigue to flu-like symptoms, potentially escalating to more severe conditions. ICANS can manifest as confusion or delirium.
In the event of CRS during treatment, it is crucial to remain calm, as most cases are manageable. Physicians typically assess the severity of CRS through grading (ranging from 1 to 4) and closely monitor the patient before implementing appropriate treatment measures. Supportive care, such as antipyretics and anti-inflammatory drugs, is commonly administered. Severe cases may require medications like tocilizumab to modulate the immune response. ICANS clinical presentations are diverse, with the majority being reversible. Close monitoring and comprehensive examinations, often utilizing the CARTOX-10 and ICE scoring systems, aid in grading and treating ICANS. In severe cases, corticosteroids may be employed. Developing an individualized treatment plan adjusted to the patient's specific condition is paramount for effective adverse reaction management.
Efficacy assessment and precautions after CAR-T therapy
The first efficacy evaluation will be performed 1 month after CAR-T cell infusion, and then at 2 to 3-month intervals thereafter. Response assessment is performed per the 2016 IMWG response criteria, which is divided into traditional response criteria and MRD response criteria. Traditional response assessment is performed first, and minimal residual disease (MRD) testing will be performed if the patient achieves complete response (CR).
Achieving negative MRD implies better progression-free survival and overall survival1 and achieving a deep response (≥ CR and MRD negative) significantly improves the prognosis. The deeper the level of response in patients, the lower the risk of relapse.
A comprehensive long-term follow-up is imperative after CAR-T therapy. The focus remains on immune monitoring, regularly assessing blood parameters and immune function to ensure the sustained efficacy of CAR-T cells and prevent potential immune-related complications. Close surveillance for relapse indicators, including regular tumor marker assessments and imaging evaluations, is crucial. Prioritizing the long-term physical well-being and quality of life for patients involves offering comprehensive support and care. Our medical team is committed to establishing continuous communication channels, ensuring timely addressing of potential long-term effects and post-treatment issues.


