Table of Contents
Currently, common treatment regimens for relapsed/refractory multiple myeloma (RRMM) can be divided into conventional therapies and innovative therapies. Conventional therapies mainly include IMiDs, PIs, monoclonal antibodies, etc. And innovative therapies mainly include immunotherapy, among which CAR-T cell therapy is one of the most effective methods for treating RRMM at present. Then, we will explain the differences between conventional therapies and innovative therapies
The uniqueness of innovative CAR-T cell therapy
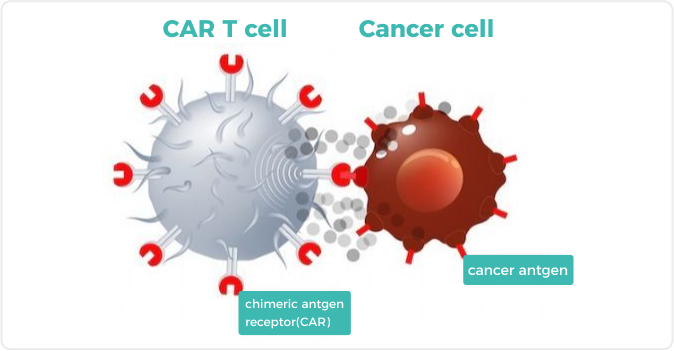
CAR-T cell therapy is a method that uses patients’ own immune T cells to fight cancer (Figure 1), and compared to conventional therapies, it usually has the following advantages:
- Precisely targets and kills multiple myeloma cells¹
- Typically requires only a single infusion, with a short treatment cycle
- Substantially prolongs the survival²
- Safe and controllable
- Persists in the body for a long time with lasting efficacy
Comparison of efficacy of CAR-T cell therapy vs. conventional therapies
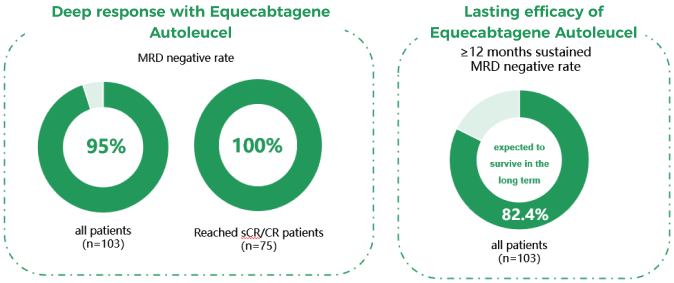
The treatment goal for RRMM is to achieve the greatest response and extend survival. Data from previous studies have clearly demonstrated that compared with other drug therapies, CAR-T cell therapy can significantly improve the response rate (Figure 2). Especially Equecabtagene Autoleucel, which is the world’s first approved fully human BCMA-targeting CAR-T for the treatment of RRMM, has achieved a total response rate of 99% in patients treated with it.
A deeper response means a longer survival and minimal residual disease (MRD) is currently one of the clinical standards for judging the degree of response, with patients achieving sustained MRD negativity expected to achieve long-term survival, and even a “functional” cure⁵. A study showed that among the population treated with Equecabtagene Autoleucel, 95% of patients reached MRD negativity, and 82.4% of patients maintained MRD negativity for ≥ 12 months, who were expected to survive in the long term⁶ (Figure 3).
Comparison of safety of CAR-T cell therapy vs. conventional therapies
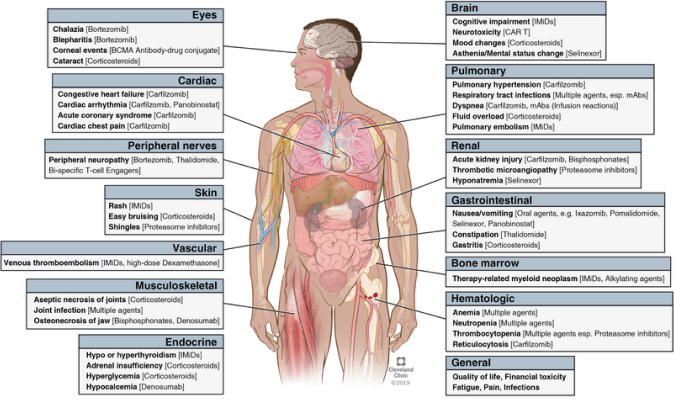
When choosing the appropriate treatment regimen, it is necessary to consider drug toxicity to avoid serious consequences caused by adverse reactions. Then, we compared the common adverse reactions during the treatment with conventional therapies and CAR-T cell therapy. In conventional therapies, different drugs cause different adverse reactions, often involving multiple systems of the body⁷ (Figure 4).
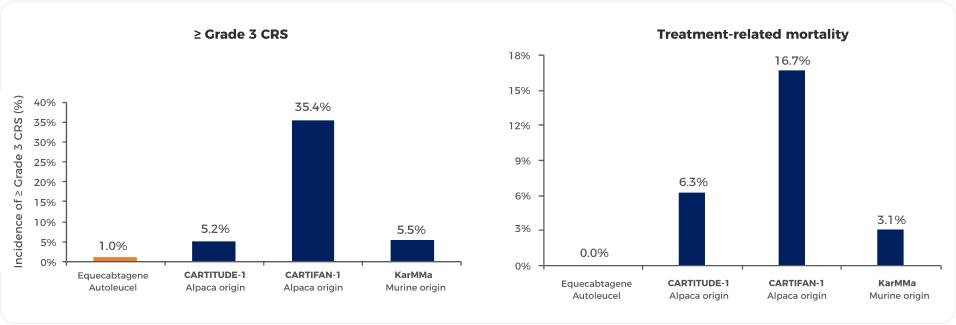
Common adverse reactions caused by CAR-T cell therapy are mainly divided into three categories: cytokine release syndrome (CRS), neurotoxicity, and infections. The most common adverse reaction after CAR-T therapy is CRS, with an incidence of up to 90%, and the incidence of ≥ Grade 3 CRS is around 5%. Pyrexia is a common first manifestation. Studies have shown that the adverse reactions of Equecabtagene Autoleucel are controllable, and the incidence of ≥ Grade 3 CRS is 1% 6 (Figure 5 4,9-11). Neurotoxicity presents in various forms, with an incidence of 10% to 42%, and the incidence of ≥ Grade 3 neurotoxicity is only 1% to 3%. The incidence of various types of infections after CAR-T therapy is about 42%, with ≥ Grade 3 infections at about 14%. Most are bacterial infections, mainly occurring within 2 weeks after cell infusion7,8.
Comparison of quality of life of CAR-T Cell Therapy vs. conventional therapies
Besides efficacy and safety, another key focus is how to enhance the well-being of patients during treatment. We have found that the burden of drug therapies is closely related to poorer compliance, economic burden, treatment stress, and even efficacy 12, hence the clinical practice has always been committed to finding treatment methods that can effectively and sustainably alleviate the burden on patients and their dependents.
Compared to CAR-T cell therapy, conventional therapies require a long duration of treatment and complex medication regimens, bringing significant economic, psychological, and clinical visit pressures to patients (Figure 6).

While CAR-T cell therapy typically requires only a single infusion, has a short treatment cycle, and can reduce clinical visit pressures, thus improving quality of life. Studies indicate that quality of life is significantly and durably improved after CAR-T therapy. Treatment for one month can significantly improve pain and MM symptoms, and treatment for two months can significantly improve fatigue, physical function, cognitive function, and overall health status/quality of life (Figure 7) 13.
Accessible CAR-T cell product for RRMM patients— Equecabtagene Autoleucel
Considering the aforementioned benefits including significant efficacy, lasting effect, controllable safety, and better quality of life, CAR-T cell products are the preferred treatment for RRMM patients currently. Among them, Equecabtagene Autoleucel, as the world’s first approved fully human BCMA-targeting CAR-T for the treatment of RRMM, has unique advantages over other CAR-T cell products, making it a standout in the field of CAR-T cell products.
Equecabtagene Autoleucel, as the world’s first approved fully human BCMA-targeting CAR-T for the treatment of RRMM, has unique advantages. The fully human BCMA-targeting CAR-T cell product, compared to animal-derived products, has stronger and deeper tumor-killing capabilities, longer persistence in the body, and can reduce immunogenicity, making it safer and more reliable (Figure 8) 14. 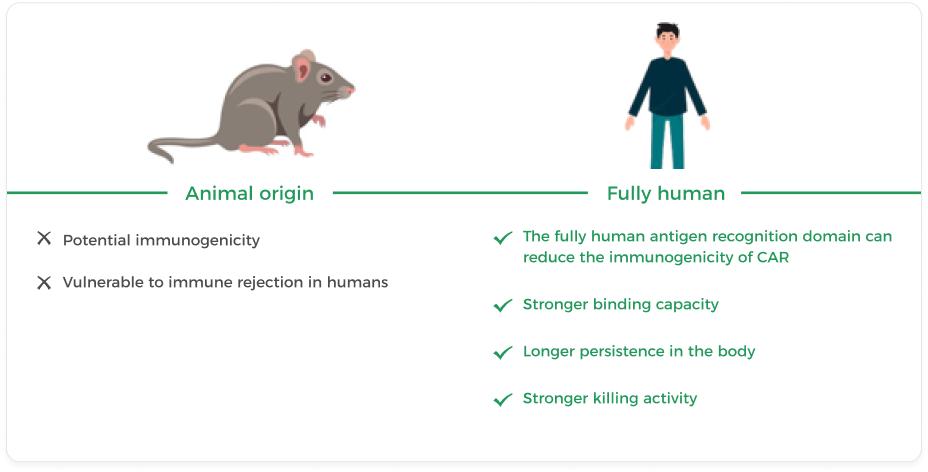
Equecabtagene Autoleucel—a personalized Living Drug, how is it prepared?
First, T cells are isolated from the patient’s peripheral blood, then transformed into CAR-T cells with strong specificity and killing action through in vitro genetic technology, and finally reinfused into the patient’s body to precisely and persistently clear myeloma cells (Figure 9) 15.
View detailed treatment plan- The Chinese consensus for the CAR-T cell therapy in multiple myeloma (2022 version).
- Wang D, et al. Blood. 2021 May 27;137(21):2890-2901.* phase 1 study of CT103A.
- Jeryczynski G, et al. Cancers (Basel). 2021 Nov 23;13(23):5886.
- 2023 IMS. P-290.
- Landgren, et al. JCO2200622. 30 Jun. 2022.
- Chunrui Li, et al. 2023 ASCO
- Chinese Medical Doctor Association, Hematology Branch, et al. Chinese Journal of Hematology, 2022, 43(04):265-271.
- Leukemia and Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association, et al. Chinese Journal of Hematology, 2022, 43(2):6.
- Lancet. 2021 Jul 24;398(10297):314-324.
- Mi JQ, et al. J Clin Oncol. 2023 Feb 20;41(6):1275-1284.
- Munshi NC, et al. N Engl J Med. 2021 February 25;384:705-716.
- Hagendorff A, et al. Adv Ther. 2013;30(4):406-19.
- Delforge M, et al. 2022 Feb 22;6(4):1309-1318.
- Rui Cui, et al. Chinese Journal of Hematology, 2021, 42(6) : 502-507.
- De Marco RC, et al. 2023 Mar 27;24(7):6300.
References:
