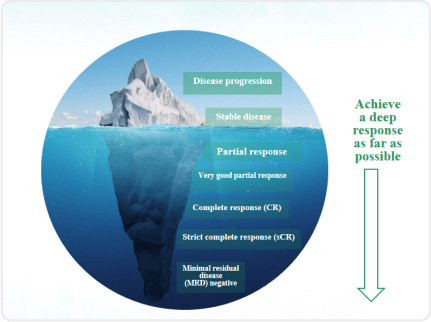
The treatment response depth and the level of minimal residual disease (MRD) play a crucial role in determining the prognosis of multiple myeloma (MM) patients. The primary treatment objective is to attain a profound response, especially achieving MRD negativity, to mitigate disease-related complications. This approach not only enhances survival rates but also elevates the overall quality of life for patients. Minimal residual disease (MRD) is characterized by the presence of undetectable residual tumor cells in the body, surpassing the capabilities of conventional methods such as morphological assessments post-hematologic complete response in treated hematologic malignancies. The persistence of negative MRD following complete response serves as a predictive factor for long-term survival, marking a significant step towards achieving a "functional" cure.
1.Chinese Hematology Association, Chinese Society of Hematology. Chinese Journal of Internal Medicine, 2022, 61(05):480-487. Plasma Cell Disease Group, Chinese Society of Hematology, Chinese Medical Association; Chinese Myeloma Committee-Chinese Hematology Association. Guidelines for the diagnosis and management of first relapsed multiple myeloma in China (2022) [J]. Chinese Journal of Hematology, 2022, 43(10): 810-817.
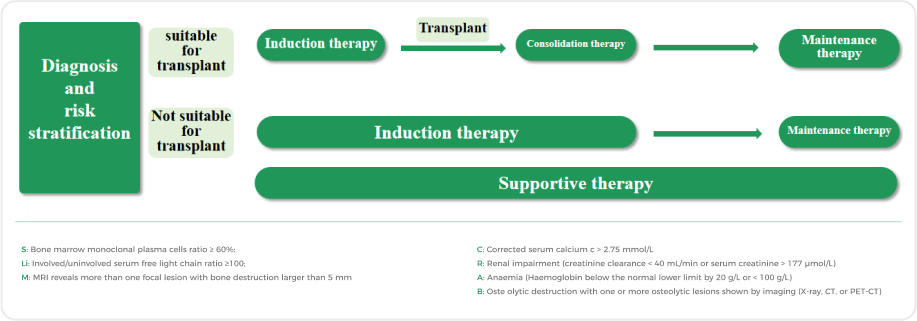
When MM patients present with CRAB or SLiM symptoms, treatment should be initiated
1.Chinese Hematology Association, Chinese Society of Hematology. Chinese Journal of Internal Medicine, 2022, 61(05): 480-487.
Patients with multiple myeloma may encounter various challenges: as the disease recurs, treatment options gradually diminish; with an increasing number of relapses, both progression-free survival and post-relapse survival tend to shorten; for those experiencing multiple relapses, the likelihood of high-risk cytogenetics rises, posing higher treatment complexities. These factors necessitate a more comprehensive and individualized approach from the medical team in navigating the intricate treatment landscape of multiple myeloma, ensuring more effective disease management and enhancing the overall quality of life for patients.
1.Ailawadhi S, et al. Mayo Clin Proc 2023. Treatment of Relapsed Myeloma.
2.Chinese Hematology Association, Chinese Society of Hematology. Chinese Journal of Internal Medicine, 2022, 61(05): 480-487.
1.Jeryczynski G, Bolomsky A, Agis H, Krauth MT. Stratification for RRMM and Risk-Adapted Therapy: Sequencing of Therapies in RRMM. Cancers (Basel). 2021 Nov 23;13(23):5886.
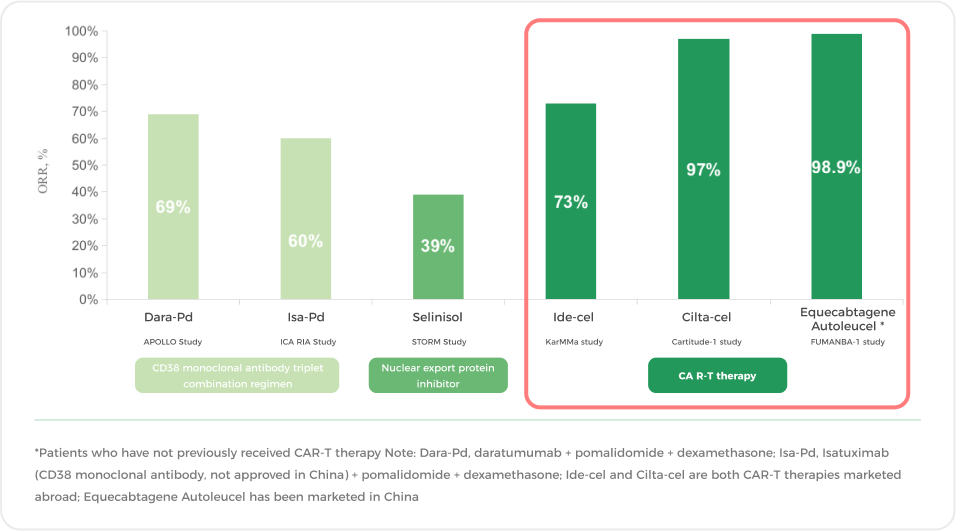
Apart from participating in clinical trials, clinicians will consider the disease condition, the efficacy of existing drugs, etc., to choose suitable new drugs or therapies for you. CAR-T therapy is one of the important options for patients with multiple relapses, especially for those with multi-drug resistance. If you are still responsive to some novel drugs, you can also try switching to new-generation drugs with different mechanisms of action or other new drugs, such as selinisol.
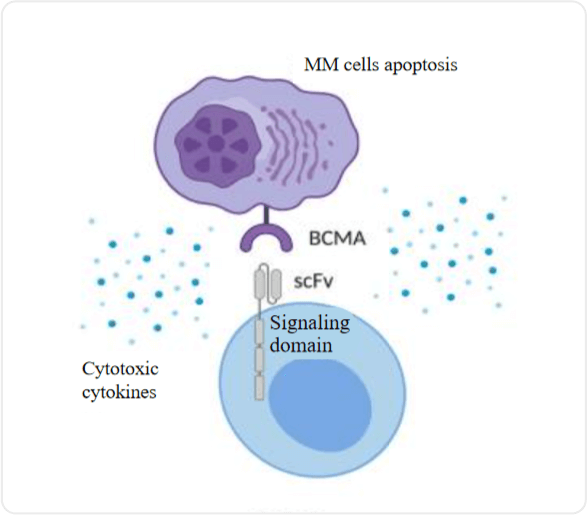
1.Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020 Sep 17;13(1):125. 2. The Chinese consensus for the CAR-T cell therapy in multiple myeloma (2022 version)
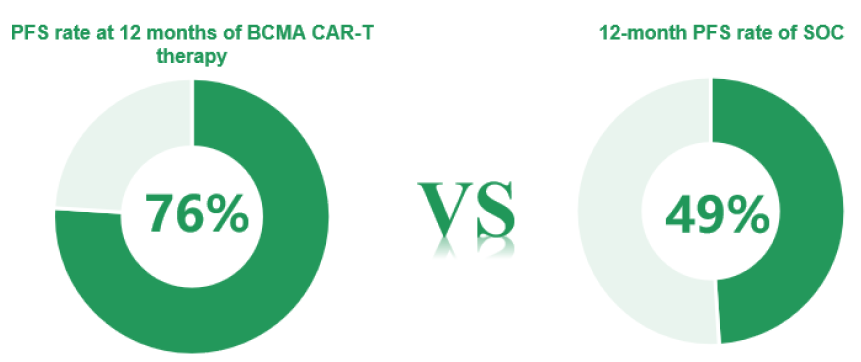
As of November 1, 2022, the median follow-up was 16 months Compared with standard of care, BCMA CAR-T significantly improved ORR, ≥ CR rate, and overall MRD-negative rate (P < 0.0001 for all)
- CARTITUDE-4 is a global, phase 3, randomized controlled study comparing targeted BCMA CAR-T cell therapy with SOC(pomalidomide, bortezomib, and dexamethasone [PVd] or daratumumab, pomalidomide, and dexamethasone [DPd]) for lenalidomide (len)-refractory MM patients (previously treated with 1-3 lines of therapy), with a total of 419 patients enrolled. The primary endpoint was progression-free survival (PFS) in the intention-to-treat population.
- Binod Dhakal, Kwee Yong, Simon J. Harrison, et al. First phase 3 results from CARTITUDE-4: Cilta-cel versus standard of care (PVd or DPd) in lenalidomide-refractory multiple myeloma. The Annual Meeting of the ASCO. Abstract LBA106.
Understanding the patient's needs, the early application of CAR-T therapy is crucial for enhancing the treatment efficacy of multiple myeloma. By employing CAR-T therapy at an early stage, we can significantly increase the response rate, reducing the risk of disease recurrence. Moreover, CAR-T therapy exhibits prolonged effectiveness, requiring only a single injection to exert its effects continuously within the body, eliminating the need for ongoing drug administration and providing a more convenient treatment option for patients.
Furthermore, early adoption of CAR-T therapy yields substantial survival benefits. Utilizing CAR-T therapy in the early stages enables us to more effectively inhibit the growth of abnormal plasma cells, enhancing the overall treatment outcomes. This implies that patients facing multiple myeloma have a greater chance of achieving sustained remission, thereby slowing down the progression of the disease.
In summary, the early application of CAR-T therapy not only improves patients' survival outcomes and treatment efficacy but also alleviates the treatment burden. We aim to offer you a more convenient and effective treatment choice. When devising your treatment plan, please keep us informed about the potential application of CAR-T therapy. We hope to bring you more positive treatment outcomes and survival benefits.
Commercial CAR-T therapy offers a reliable treatment option for patients with relapsed or refractory multiple myeloma (RRMM). In comparison to CAR-T clinical trials, commercial CAR-T therapy demonstrates clear advantages. In clinical trials, patients need to wait for enrollment opportunities, and the screening criteria for enrollment are stringent. Even if eligible, the waiting period for enrollment can be several months or up to a year. Additionally, due to the product not being on the market or not approved for a specific indication, its efficacy and safety are not fully validated. Commercial CAR-T therapy, on the other hand, is more convenient, allowing patients to participate if they meet the indication and physical criteria, without prolonged waiting. Furthermore, this therapy undergoes rigorous scrutiny before being commercially available, ensuring both efficacy and safety. The entire production process adheres to Good Manufacturing Practice (GMP) standards and regulatory oversight, meeting or exceeding national standards, providing patients with a trustworthy treatment assurance.
1.Good Clinical Practive: https://www.gov.cn/gongbao/content/2020/content_5525106.htm?ivk_sa=1024320u
-- The World's First Approved Fully Human CAR-T Therapy
- Full epitope of light and heavy chains binds tightly to BCMA
- Fast dissociation, low exhaustion
- Low immunogenicity
- Median duration of persistence time 419 days
- 12-month sustained MRD negativity rate 81.7%
- 12-month PFS rate 85.5%
- ORR 98.9%
- ≥CR rate 82.4%
- MRD negativity rate 97.8%
- No ≥ Grade 3 ICANS
- Incidence of ≥ Grade 3
- No movement/cognitive disorder observed No Parkinson's disease found
We use cookies to ensure that you get the best experience on our website. Clicking "Accept Cookies" means you agree with our Privacy Policy.
Accept Cookies
.png)
.png)
.png)
.png)
.png)

