Lu Daopei Hospital specializes in hematology. The hospital is named after Dr. Lu Daopei who is a renowned hematology specialist and pioneer of Chinese bone marrow trans plantation, and also an academician at the Chinese Academy of Engineering.
Currently we have 2 hospitals in China which is Hebei Yanda Lu Daopei Hospital and Beijing Lu Daopei Hospital. Our clinical and research facilities provide full-suite of diagno sis and treatment for various blood diseases. Boast hematopoietic stem cell transplanta tion(HSCT), CAR-T, and treatment for leukemia, lymphoma and myeloma.
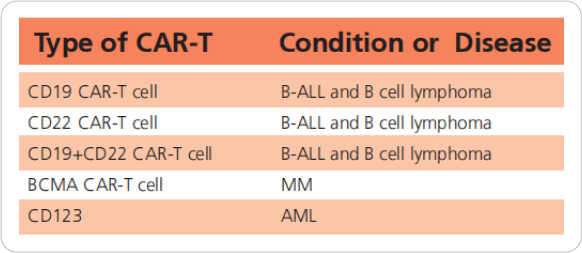
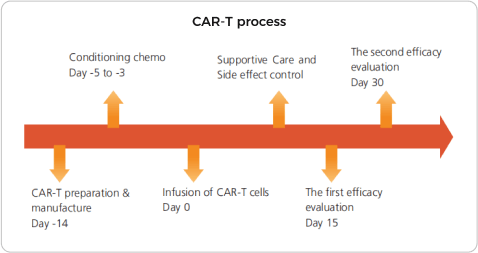
Until 2018, more than 500 patients have participated in the CAR-T clinical trial in our hos pital. The complete remission rate is about 90%. We have developed the technology of performing CAR-T timely bridging to allo-HSCT, which could overcome the risk of relapse and further improve long term survival rate.
As one of the most active HSCT centers, our HSCT department performs 1/10 of the total HSCT cases in China every year. In 2018, we completed HSCT 773 cases, including 546 haplo-HSCT cases.
The International Center provides personalized and culturally appropriate support starting from the initial correspondence until the day each patient returns home. Our goal is to make it easy for patients to focus on getting better and feeling well.
By December 2018, Lu Daopei Hospital has attended over 500 patients by using CAR-T technology in
clinical trials. And the CR rate is 90%.
We can offer different clinical trials for different diseases.
Beijing Lu Daopei Hospital is located in Yizhuang Economic Development Zone,with an area of 27,000 m²,300 beds and 36 laminar air flow rooms.