Beijing GoBroad Boren Hospital of GoBroad Medical Institute of Hematology(Beijing Center), affiliated with GoBroad Healthcare Group, was established in Beijing in 2017 as the first flagship hospital. It serves as the center for the diagnosis and treatment of difficult hematology &oncology cases within the Group and is a secondary comprehensive hospital designated by the Beijing Municipal Basic Medical Insurance. The hospital is located by the South Third Ring Road, Fengtai District, Beijing, with a building area of 14,100 square meters and 170 authorized beds. It also features a modern Phase I Clinical Trial Center built according to international standards.
Construction – Immunotherapy
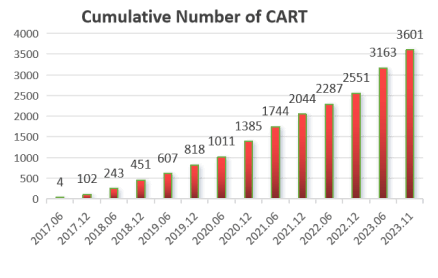
From June 2017 to December 2022, a total of 3,601 cases of CAR-T clinical study have been completed, with a complete remission rate of over 80%.
The adverse reactions of CRS are controlled at grade 2 or below, and clinical management of neurotoxicity is proficiently handled, professional AE/SAE medical judgments.