What is FUCASO® (Equecabtagene Autoleucel Injection)?
FUCASO® (Equecabtagene Autoleucel Injection) was approved by the NMPA on June 30, 2023, for the treatment of adult patients with relapsed and/or refractory multiple myeloma (r/rMM) who have progressed after at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.

How FUCASO® works?
FUCASO® is a gene-modified autologous T-cell immunotherapy designed to target BCMA (B-cell maturation antigen), enabling it to recognize and eliminate malignant cells expressing BCMA. The CAR (chimeric antigen receptor) in FUCASO® is composed of a fully human single-chain antibody fragment that specifically binds to BCMA. Upon binding to BCMA-positive cells, the CAR transmits signals that promote CAR-T cell proliferation and activation, leading to the release of inflammatory cytokines and the targeted killing of malignant cells.
Unlike other conventional cancer treatments, such as chemotherapy, FUCASO® is made from your own white blood cells that have been genetically modified to recognize and attack your multiple myeloma cells.
Unique Advantages of FUCASO®
Fucaso® has breakthrough fully human CAR structure, full epitope binding to BCMA, low exhaustion, strong efficacy
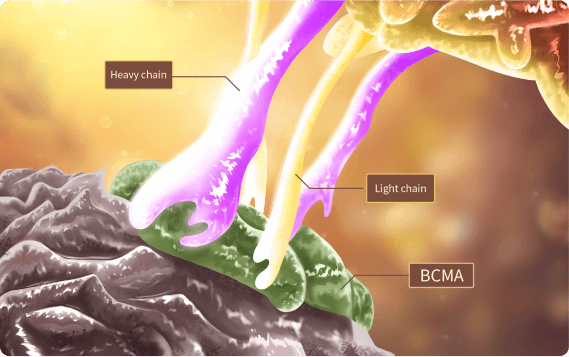
*There is a natural interaction between the light and heavy chains to form a stable structure, and the epitope completely covers BCMA
Dynamic model of the binding of fully human scFv to BCMA antigen

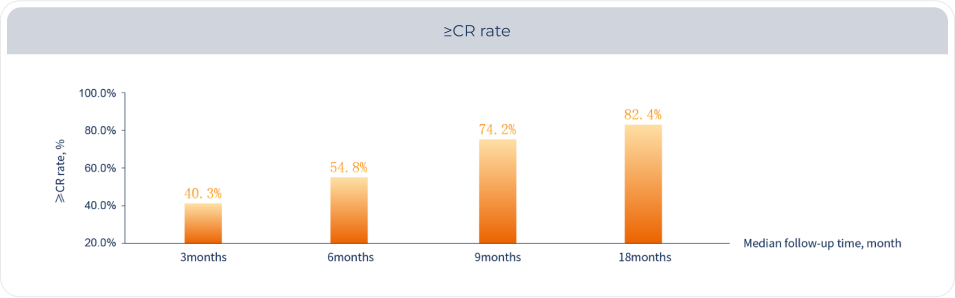
Fucaso® demonstrates strong efficacy, achieving a ≥CR rate of up to 82.4%
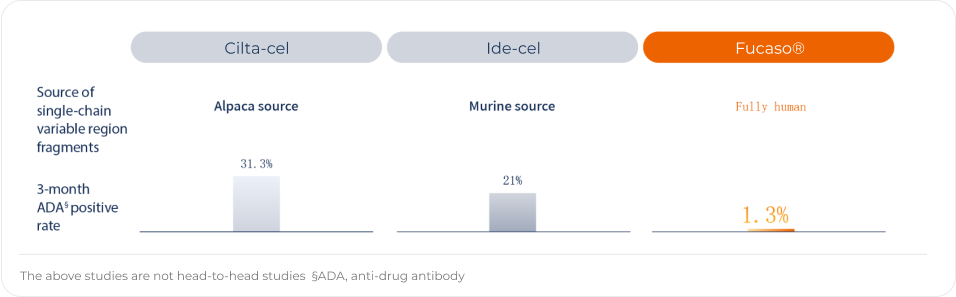
Fucaso® has breakthrough fully Human CAR structure, low immunogenicity, and long-term persistence
Fucaso® utilizes fully human scFv with low immunogenicity, making it less likely to develop anti-drug antibodies (ADAs) that affect CAR-T cell survival in the body after infusion
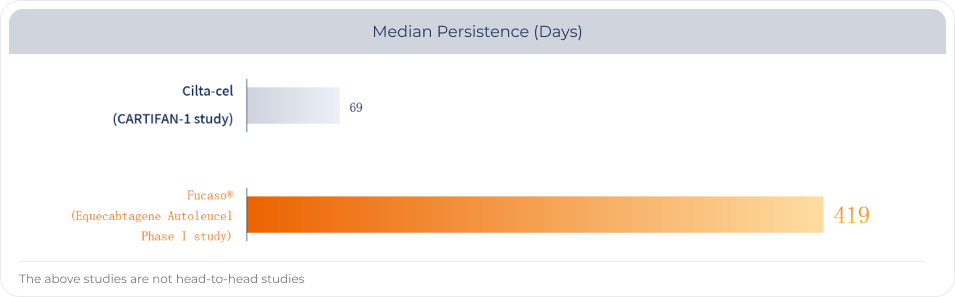
Fucaso® has a median in vivo persistence of over one year
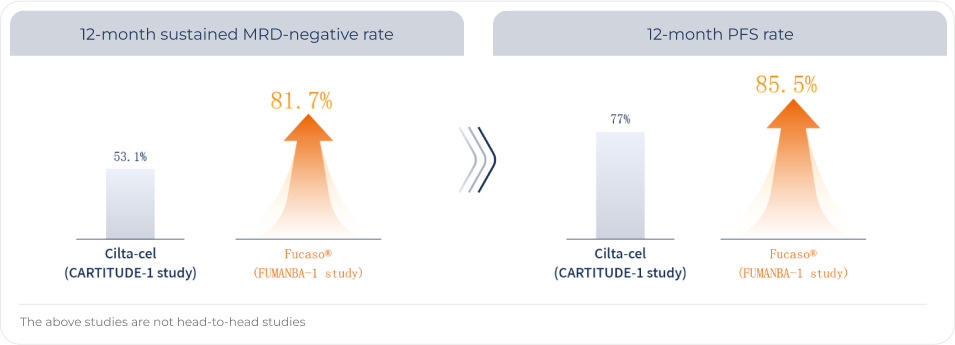
For Fucaso®, the sustained MRD-negative rate at 12 months was up to 81.7% PFS rate at 12 months was up to 85.5%
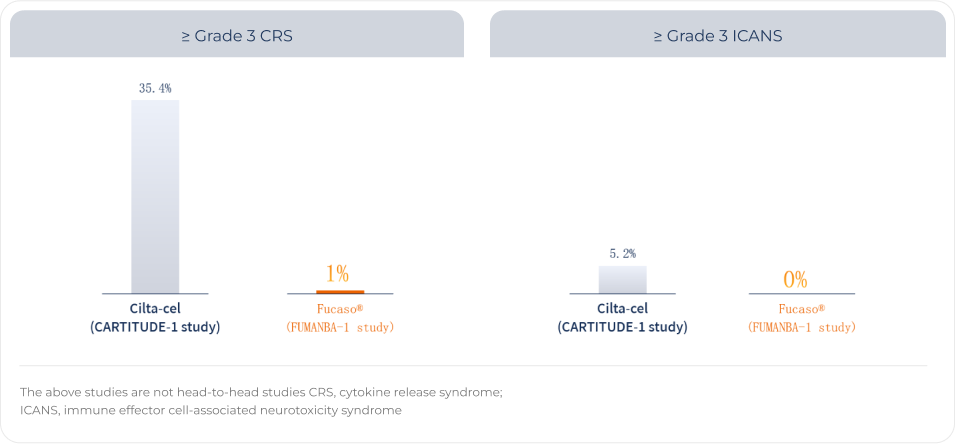
Favorable Safety Profile of Fucaso®
Patients receiving Fucaso® had no incidence of ≥ Grade 3 ICANS, and an incidence of ≥ Grade 3 CRS of only 1.0%
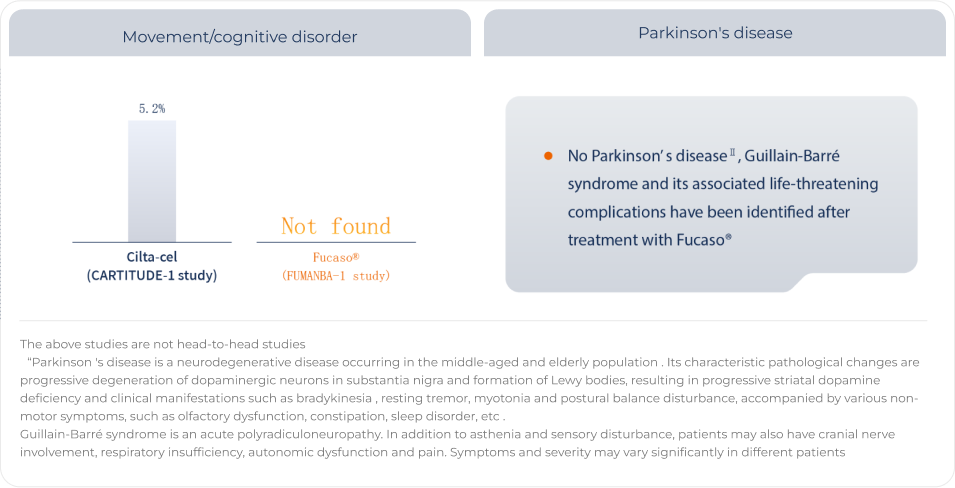
No movement/cognitive disorder or Parkinson's disease was found in patients receiving Fucaso®
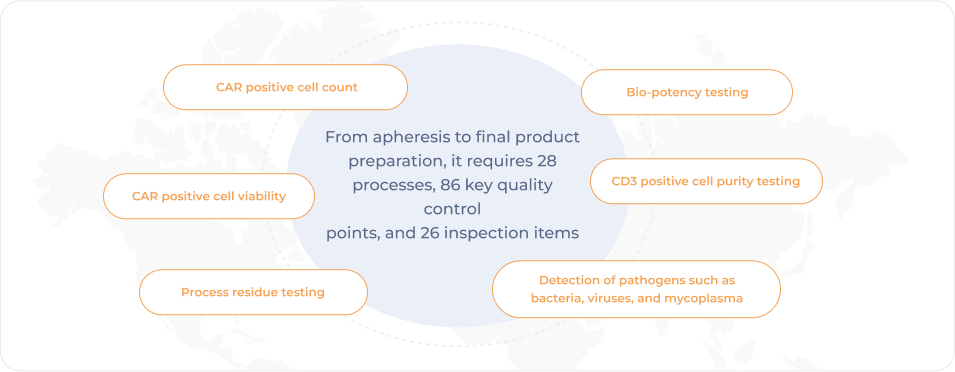
Full process of Fucaso® complies with GMP system requirements and regulations
Multiple-dimension release testing to ensure compliance with strict requirements of national regulations for the commercialization of CAR-T
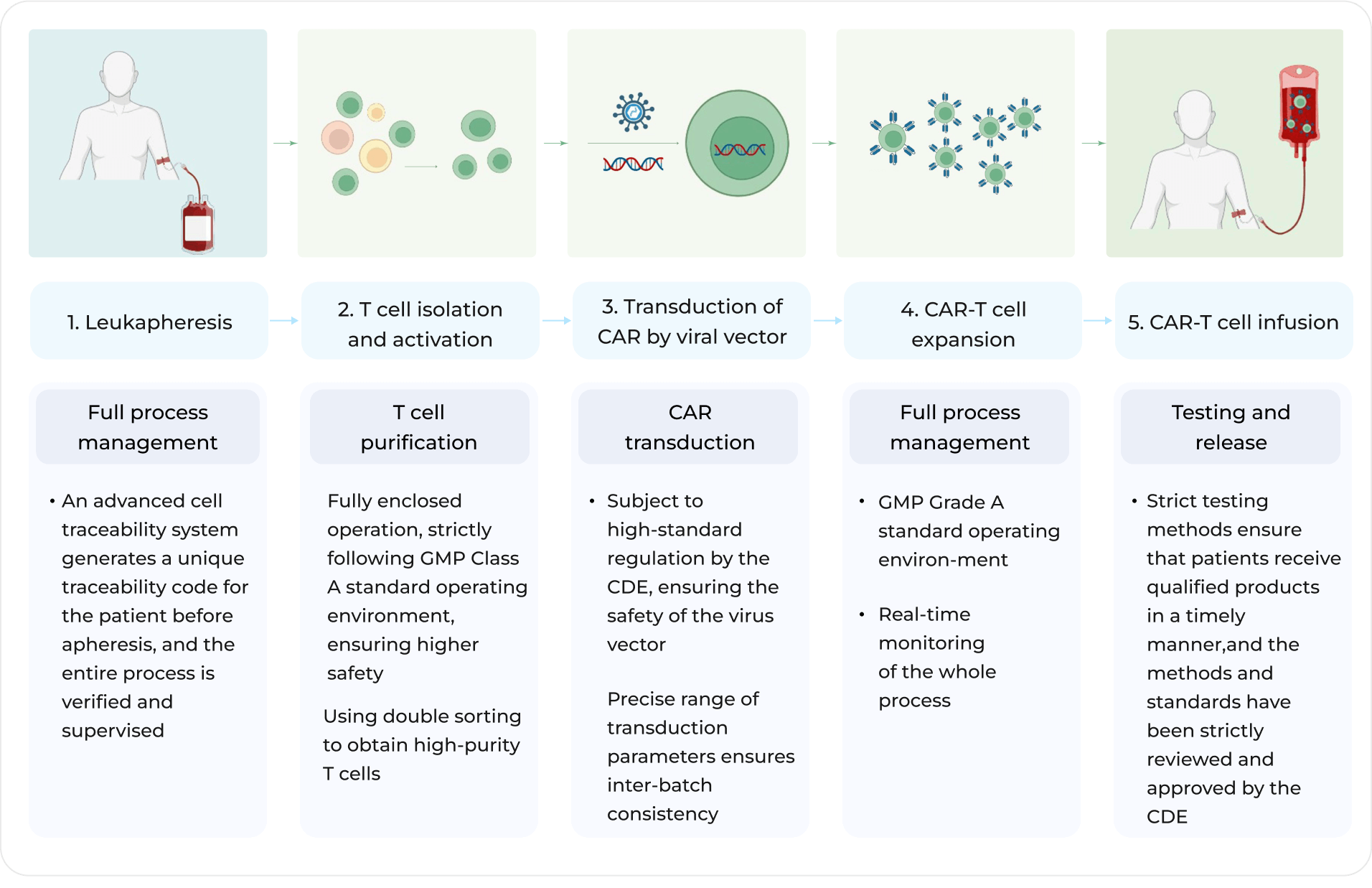
The production process of Fucaso® strictly complies with GMP requirements to ensure high-standard and high-quality release
*Study Introduction

*Resource
Global Recognition of FUCASO®

-- Recommended by three major global guidelines
- Consensus guidelines and recommendations for the management and response assessment of chimeric antigen receptor T-cell therapy in clinical practice for relapsed and refractory multiple myeloma: a report from the International Myeloma Working Group Immunotherapy Committee
- Guidelines of Chinese Society of Clinical Oncology (CSCO) for CAR-T Cells in the treatment of Hematological Malignancies, 2024
- The Chinese consensus for the CAR-T cell therapy in multiple myeloma(2022 version)
Who is FUCASO® for?
If you're considering FUCASO®, as a personalized treatment, you will need to be evaluated by a certified treatment center, where a professional medical team will determine your eligibility for FUCASO® and, if suitable, prescribe and administer the therapy. Our medical team will assist you with detailed consultations and evaluations and will remain actively involved in your care throughout the entire process.
Click to start your consultation and evaluation
The FUCASO® Treatment Process
FUCASO® differs significantly from traditional cancer treatments like chemotherapy and radiation. This therapy uses T cells extracted from your own blood, which are genetically engineered to incorporate a chimeric antigen receptor (CAR) gene targeting B-cell maturation antigen (BCMA). This innovative approach allows FUCASO® to precisely identify and attack multiple myeloma cells in your body, offering a unique treatment option.
The FUCASO® treatment is a 6-step process that typically takes 2 to 3 months to complete.
CELL COLLECTION
~3 TO 6 HOURS
GENETICALLY MODIFYING YOUR T CELLS
~4 TO 5 WEEKS*
PRE-INFUSION TREATMENT
3 DAYS
ONE-TIME FUCASO® INFUSION
~30 TO 60 MINUTES
Short-Term Monitoring
Up to Day 28
Long-Term Monitoring
After Day 28

Some of your blood is drawn into a machine that separates the white and red blood cells, collects some of the white blood cells (including T cells), and returns the rest of the blood into your body. This process is called leukapheresis (loo-kuh-fur-ee-sis). This process may take ~3 to 6 hours and may need to be repeated until the process is complete.
Your white blood cells are frozen and sent to a manufacturing site, where the T cells are separated out and customized into your FUCASO® CAR-T cells. This is done by genetically modifying your T cells to be able to recognize BCMA on the surface of multiple myeloma cells. Your FUCASO® CAR-T cells are then frozen and sent to your FUCASO® Certified Treatment Center.

A few days before your infusion of FUCASO®, you’ll receive low-dose chemotherapy infusions with cyclophosphamide and fludarabine. These infusions will help prepare your body for the CAR-T infusion. Each of these infusions will be given to you once a day for 3 days. These infusions are given to help clear out some of your white blood cells to make the necessary space in your immune system for FUCASO®. This is also known as lymphodepleting chemotherapy.
About a month after your initial cell collection, and 2 to 4 days after your last low-dose chemotherapy, you’ll be given your FUCASO® through a one-time intravenous infusion that takes approximately 30 to 60 minutes. Your healthcare provider will guide you through what your infusion day will be like.

After receiving FUCASO® treatment, your healthcare team at the treatment center will closely monitor you for any signs of treatment reaction during the first 14 days post-infusion. It’s recommended that you stay near the treatment center for the first 4 weeks so your healthcare team can regularly check on your progress and assist with any side effects. If severe side effects occur, hospitalization may be necessary until they are under control and it is safe for you to be discharged. During this period, your healthcare provider will conduct blood tests to track your progress. It’s crucial to keep these appointments. If you miss one, contact your provider to reschedule as soon as possible

Following the initial 4-week monitoring period, your healthcare team will continue to provide care and work with you to develop a plan for long-term monitoring and regular follow-ups. Stay in communication with your healthcare team, especially if you experience any discomfort or changes in your health. Notify your healthcare team immediately if anything feels off.
Get Ready for FUCASO Treatment
Traveling to China for medical care can present financial and logistical challenges, so we offer a range of support options, including free online consultations, logistics services, private/public hospital appointment services, CAR-T treatment, medical translation, medical accompaniment, and family arrangements, ensuring that patients receive a seamless and high-quality treatment experience.
Contact us for support
Resources

Equecabtagene Autoleucel (FUCASO)Clinical Facts and Clincal Handling Guidance

Your guide to Equecabtagene Autoleucel( FUCASO )
For the treatment of adult patients with relapsed or refractory multiple myeloma.

Witness the powerful account of life’s miracle, meticulously created by Fucaso.
View Miracle Stories