Table of Contents
Prof. Qiu Lugui, Yi Shuhua, An Gang, Yu Tengteng: Breaking the situation and reborn, Equecabtagene brings hope of cure to ultra-high-risk R/RMM.
Multiple myeloma (MM) is a malignant proliferation of clonal plasma cells, which is still incurable. In recent years, new drugs have been introduced to improve the prognosis of MM, and the median overall survival (OS) of MM is now close to 10 years1.
However, the prognosis of high-risk MM is still not optimistic. The median OS of high-risk MM (HRMM)is less than 3 years2, and the prognosis of ultra-high-risk MM patients (with ≥2 high-risk cytogenetic abnormalities) is worse3.Therefore, for ultra-high risk relapsed refractory MM (R/RMM) patients, there is still a need to seek better treatment strategies.
In this issue, Professor Yu Tengteng of Hematology Hospital of Chinese Academy of Medical Sciences is invited to share a case of Equecabtagene in treating ultra-high-risk MM patients.
Relapse after 4 drugs plan, what treatment plan does ultra-high-risk R/RMM still have?
CAR-T therapy is a better solution for ultra-high-risk R/RMM
Patient’s general conditions
The patient, a 51-year-old female, visited another hospitalin November 2022 due to “fatigue for half a year accompanied by chest pain and abdominal distension”. After examination of rib fracture and anemia and splenomegaly, she was transferred to another hospital and received relevant examinations.
Bone marrow smear: plasma cell ratio 59%; bone marrow pathology: plasma cell myeloma with secondary bone fibrosis; blood IFE: IgA κ positive; urine IFE: IgA κ positive, diagnosed as MM. After 2 cycles of VAD treatment locally, she visited our hospital. ECOG score of 0.
VAD: bortezomib + doxorubicin + dexamethasone
Patient baseline examination
Blood routine: WBC 3.69×10 9/L, RBC 3.14×10 12/L, HGB 88g/L, PLT 187×10 9/L.
Hemochemistry: ALB 33.30g/L, GLB 33.60g/L, ALT 10.80U/L, AST 21.80 U/L, Cr 45.70umol/L, β2-MG 3.93mg/L, LDH 349.90U/L.
Blood M protein identification: immunoglobulin A 8.88g/L↑, light chain κ 1240.00mg/dl, light chain λ 415.00 mg/dl, κ-FLC 65.02mg/L↑;λ-FLC 12.05mg/L;rFLC 5.40;dFLC 52.97mg/L; blood immunofixation electrophoresis showed a monoclonal IgA κ component in β region.
Urine M protein identification: urine protein negative; β2-MG 0.19mg/L; light chain κ 1.85mg/dl; light chain λ 5.00mg/dl; urine immunofixation electrophoresis negative.
Bone marrow morphology examination: triple hyperplasia bone marrow image.
Bone marrow biopsy: plasma cell myeloma with bone marrow fibrosis.
Immunohistochemistry: CD138 +, MUM1 +, κ+, λ-, CD56 partial +, CD20-, CD3-.
Genetic test: IGH/MAF gene rearrangement positive (t (14;16)), is polyploid positive; IGH/MYC gene rearrangement positive (t (8;14), is polyploid positive, 1q 21+.
Imaging examination: uneven bone density and multiple low density shadows, pathological fracture of right ilium; splenomegaly, large low density shadows in parenchyma.
Clinical diagnosis
MM[IgA κ, DS stage IIIB, ISS stage II, R-ISS stage III;1q21 +;t (14;16);t (8;14)]
Diagnosis and treatment *
| Time period | Programme | Adverse reactions and comments | Efficacy |
|---|---|---|---|
| 2022-11 | VAD*2, Dara-VAD*4 | – | PD |
| 2023-06 ~ 2023-08 | SKRd*2 | – | PD |
* Dara: daratumumab; S: sellinisox; K: carfilzomib; R: lenalidomide; d: dexamethasone; PD: progressive disease;
On 25 August 2023, the patient experienced headache, diplopia and hearing loss. Imaging examination showed abnormal signals in the skull base and skull, bilateral dural thickening, bilateral maxillary sinus, ethmoid sinus and sphenoid sinusitis; bilateral mastoiditis, right mastoid considering invasive lesions. Bone marrow biopsy and minimal residual disease (MRD) examinationdetected 11.5% plasma cells and 1.14% abnormal plasma cells respectively, suggesting central infiltration of disease progression.
Considering that the patient was at ultra-high risk of MM, the risk of relapse was high and the patient failed to achieve remission after Dara-VAD and SKRd treatment. The FUMANBA-1 study showed that Equecabtagene treatment for multiline relapsed/refractory (R/R) MM resulted in deep response, with overall response rates and MRD-negative rates of 98.9% and 97.8%, respectively, in patients without prior CAR-T treatment history. In the overall population, 80.8% of patients remained MRD-negative for more than one year4.
Based on the above background, the patient and her family decided to receive CAR-T therapy after consultation to overcome the poor prognosis of high-risk cytogenetics and achieve long-term survival.
On 15 September 2023, lymphocytes were collected to prepare BCMA CAR-T cells.
On 25 September 2023, chemotherapy with DECP regimen (cisplatin 10mg, etoposide 30mg, cyclophosphamide 0.3 g and dexamethasone 20mg) was administered.
On 18 October 2023, combination therapy with fludarabine and cyclophosphamide (fludarabine 40mg and cyclophosphamide 0.4 g on Days 1 to 3) was administered.
On 23 October, 2023, the reinfusion dose was 1.0×106/kg B cell maturation antigen (BCMA) CAR-T cells.
Four days after reinfusion, interleukin-6 (IL-6) showed a trend of increase (Figure 1), which was considered as Grade 1 cytokine release syndrome (CRS). Empirical anti-infection, symptomatic antipyretic treatment and supportive treatment were given due to neutropenia.
Fever occurred on Day 6 after reinfusion (Figure 2), which recovered after ibuprofen administration. On Day 9 after reinfusion, the maximum body temperature was 40℃, which recovered after symptomatic treatment with tocilizumab. The patient’s blood pressure and oxygen saturation were normal during post-transfusion monitoring, C-reactive protein (CRP) was highest at 31.48 mg/L, ferritin was highest at 1579.3 ng/ml, and blood culture was negative.
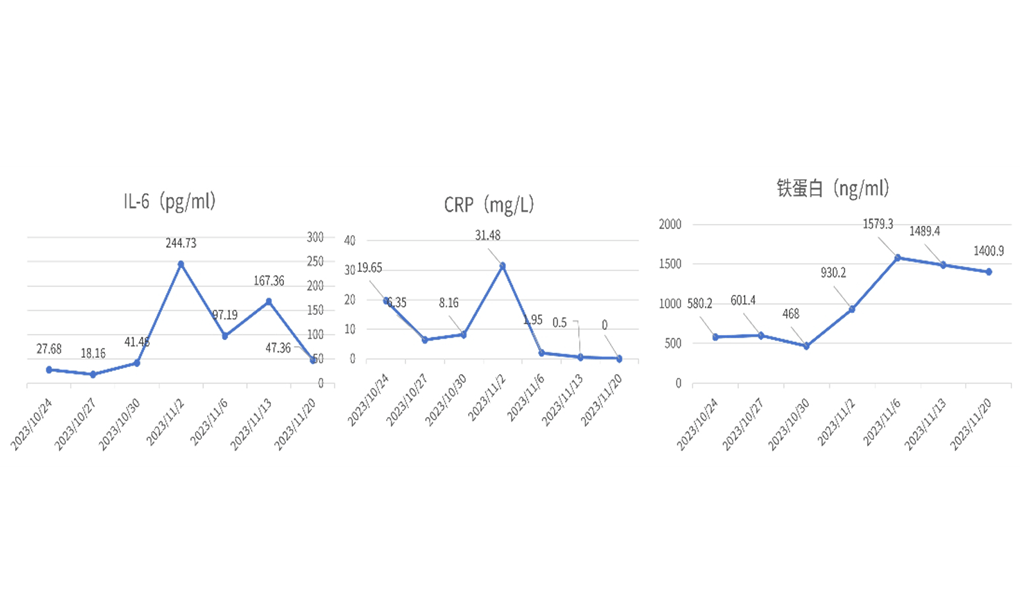
Figure 1. IL-6, CRPand ferritin contentafter CAR-T
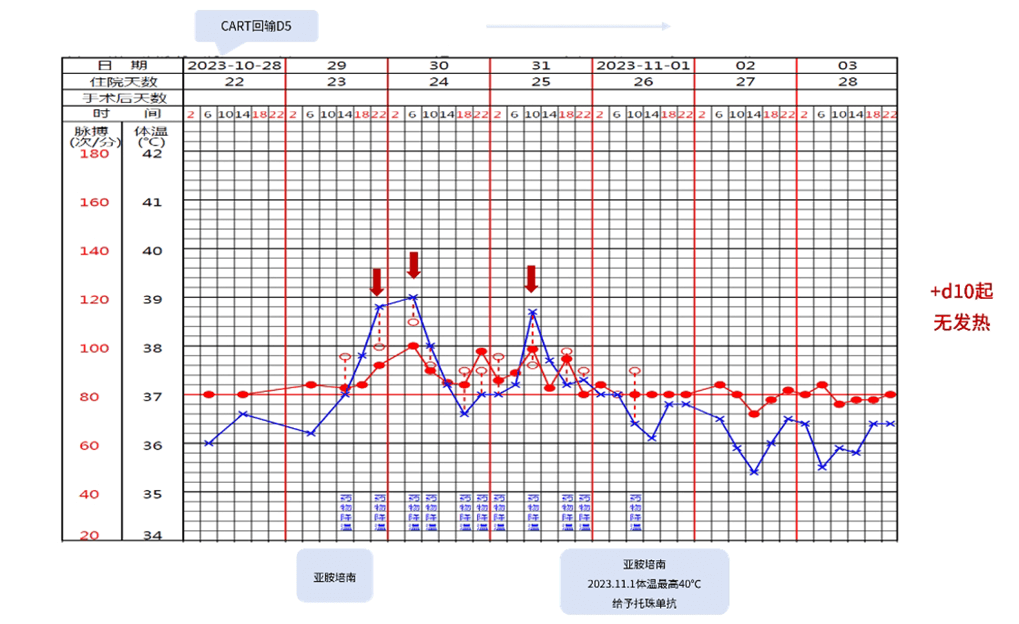
Figure 2. Temperature and pulse after CAR-T treatment
In November 2023, the patient was discharged smoothly on day 21 after the transfusion of Equecabtagene, and the efficacy was assessed as complete response (CR) in hematology and negative MRD (Figure 3). Imaging examination revealed disappearance of invasive lesions in the right papilla.
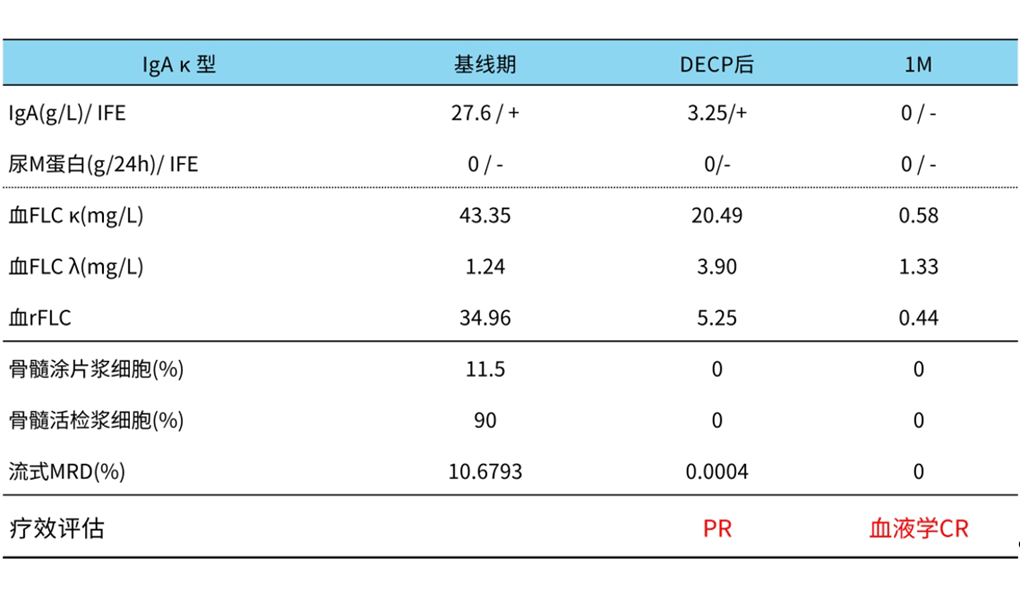
Figure 3. Patient efficacy assessment results
Professor Yu Tengteng’s Experience in Medication
This MM case was associated with 1q21 +, t (14;16) and t (8;14), belonging to ultra-high-risk MM. The patient was treated with Dara-VAD and SRD regimens, and the efficacy assessment was PD. After SRD treatment, headache, diplopia and hearing loss occurred. Imaging, bone marrow morphology and MRD examination indicated central infiltration. Chinese Guidelines for the Diagnosis and Treatment of Multiple Myeloma (revised in 2022) recommend that relapsed patients can receive new drug regimens such as CAR-T and multi-drug chemotherapy.
The FUMANBA-1 study suggests that Equecabtagene improves the depth of response and improves long-term survival in patients with R/R MM. Therefore, after consultation with the patient and her family, it was decided to administer Equecabtagene.
The patient’s efficacy assessment 1 month after reinfusion was hematologic CR and MRD negative, grade 1 CRS occurred 4 days after reinfusion, which was relieved after symptomatic treatment, and no immune effector cell-related neurotoxicity syndrome (ICANS) occurred. This case confirms that Equecabtagene has a significant efficacy and good safety profile.
For ultra-high-risk MM with central invasion and multi-line recurrence, Equecabtagene therapy is the first choice, so as to overcome the adverse prognosis of ultra-high-risk MM and improve
Comments by Professors
Ultra-high-risk MM has the characteristics of strong invasion and poor prognosis, which is the difficulty and pain point of clinical treatment at present. In the era of new drugs, four-drug regimens can improve the clinical benefit of some HRMMs6. However, this case could not be relieved and central infiltration occurred after receiving Dara-VAD and SKRd four-drug combination therapy.
The prognosis of MM with central invasion is worse, the median survival time is only 3 months7. There is no standard treatment scheme at present. The commonly used treatment schemes include intrathecal injection, local radiotherapy and systemic chemotherapy, but the treatment effect is not ideal, and the duration of treatment is short8. This suggests that this case needs to be treated with other new therapies that can achieve response faster.
The FUMANBA-1 study showed that Equecabtagene resulted in higher and longer remission rates for R/RMM4. There have been case reports showing that R/RMM with central invasion may benefit from BCMA CAR-T treatment7.This patient achieved hematologic CR and MRD negativity 1 month after receiving Equecabtagene, suggesting that Equecabtagene rapidly improves survival benefit for ultra-high-risk R/RMM with central invasion.
We look forward to the long-term follow-up results of this patient and hope that in the future, Equecabtagene will bring survival benefits to more ultra-high-risk R/RMM.
References
1. Goldman-Mazur S, Kumar SK. Am J Hematol. 2021;96(7):854-871.
2. Kaiser MF,et al. Blood 2019; 134: 604-604.
3. Zhang Enfan, Yang Li, Cai Zhen. Chinese Journal of Internal Medicine, 2020, 59(07) : 493-493.
4. Chunrui Li, et al. 2023 ASCO. Abstract 8025.
5. Hematology Society, Chinese Medical Association. Chinese Journal of Internal Medicine, 2022, 61(5):8.
6. Costa LJ, Chhabra S, Medvedova E, et al. Lancet Haematol. 2023;10(11):e890-e901.
7. Wang T, He T, Ma L, et al. Front Oncol. 2022;12:854448.
8. Li Lu, Zhang Jing, Feng Zhongyuan, et al. Leukemia Lymphoma, 2023, 32(06) : 356-359.


