Table of Contents
Achieving haematological CR in 3 weeks, Equecabtagene helps overseas patients in deep remission.
Relapsed refractory multiple myeloma (R/R MM) is a complex disease requiring different treatment strategies. In recent years, NCCN guidelines1 and Chinese multiple myeloma diagnosis and treatment guidelines2 have added CAR-T therapy to the treatment recommendations for R/R MM, which is expected to bring new breakthroughs in the treatment of these relapsed refractory multiple myeloma patients.
Professor Li Ping from the Department of Hematology, Tongji Hospital, Shanghai shared with us a successful case of R/R MM treated with BCMA CAR-T injection. The hematological complete response (CR) was achieved within 22 days after CAR-T reinfusion. Professor Liang Aibin commented on this case and shared it as follows.
Patient’s general conditions
Patient, female, 58 years old, Thai. In July 2021, multiple myeloma was diagnosed in a hospital in Thailand. After systemic treatment including ASCT, multiple relapses still occurred, drug resistance to multi-line therapy could not be effectively controlled.
On September 1, 2023, the patient was admitted to our hospital for treatment and complained of obvious pain in left shoulder and back. Previous history of restless leg syndrome, brain tumor and thyroid tumor surgery, no other underlying diseases, good health status, ECOG score of 1.
Clinical diagnosis
IgA κ MM
Process of diagnosis and treatment
| Time period | Treatment regimen | Efficacy evaluation |
|---|---|---|
| 2021-07 | PCD (bortezomib, cyclophosphamide, dexamethasone) | PR |
| 2021-10 | VRD (bortezomib, lenalidomide, dexamethasone) | SD |
| 2022-07 | DVD (CD38mAb, bortezomib, dexamethasone) | PD |
| 2022-10 | PCD (pomalidomide, cyclophosphamide, dexamethasone) | CR |
| 2023-01 | ASCT (autologous hematopoietic stem cell transplantation) | |
| 2023-05 | PCD (bortezomib, cyclophosphamide, dexamethasone) | PD |
| 2023-08 | KD (carfilzomib + dexamethasone treatment) | PD |
Admission assessment
- Serum immunofixation electrophoresis: IgA κ light chain; serum M protein: 0; urine M protein: 413.85 mg/24h
- Bone marrow abnormal plasma cells: 18%
- PET-CT showed soft tissue mass (3.5cmX2.3cm4.8cm) between the first and second ribs on the left side, multiple metabolic lesions such as sternum, ribs and spine.
- Chromosome detection: complex karyotype and del (13).
- FISH: 1q21 amplification.
The patient was relapsed refractory MM, resistant to multi-line therapy, and met the CAR-T (Equecabtagene Autoleucel) treatment indication. After multidisciplinary consultation and discussion, Professor Li Ping team of Shanghai Tongji Hospital designed individualized CAR-T treatment regimen for patients:
- Bridge therapy (KAD regimen + radiotherapy) was first performed to reduce tumor burden;
- FC (fludarabine/cyclophosphamide) regimen was followed by sequential myeloablative chemotherapy to improve CAR-T cell transfusion environment;
- And autologous BCMA CAR-T therapy was finally given (Table 2). The whole process monitoring includes CAR-T cell expansion dynamic monitoring, cytokines and organ function, etc.
| Time period | Treatment regimen | Efficacy evaluation |
|---|---|---|
| 2023-09-01 ~ | KAD programme | |
| 2023-09-24 ~ 2023-09-29 | Local radiotherapy | |
| 2023-10-09 ~ 2023-10-12 | Chemotherapeutics | |
| 2023-10-14 | CAR-T treatment | |
|
2023-11-13 2023-12-21 2024-01-10 |
Loss back 1 month | CR |
| Loss back 2 months | CR | |
| Loss back 3 months | CR |
Patient assessment before apheresis: On September 8, 2023, all test items met apheresis requirements, ECOG score was 2 points, SpO2 ≥92%, virological indicators were negative, creatinine clearance rate of renal function was>30ml/min, blood routine was normal, coagulation function was normal, and no immunosuppressant was used.
Bridging regimen: Bridging therapy started on September 11, 2023 with carfilzomib, dexamethasone + doxorubicin liposomes. Rash occurred after doxorubicin liposome, which was improved after treatment. Local radiotherapy was started on September 24 to relieve bone pain.
CAR-T Pretreatment Assessment
On October 6, imaging examination showed that extramedullary mass was larger than before, and there was new extramedullary mass, which was considered as disease progression (PD).
On October 9, bone marrow flow cytometry showed that the proportion of abnormal monoclonal plasma cells was 79.5%.
CAR-T therapy
Leucine clearance and Equecabtagene reinfusion regimen: Fludarabine + cyclophosphamide leucine clearance chemotherapy on October 9-12, and CAR-T cell reinfusion on October 14.
Safety monitoring: fever occurred on D5 after CAR-T reinfusion, with the highest temperature of 39.4°C, and significant increase of IL-6 and CRP occurred on D6. Cytokine release syndrome (CRS) grade1 was considered, and symptoms were improved after tocilizumab. The temperature returned to normal on D9.
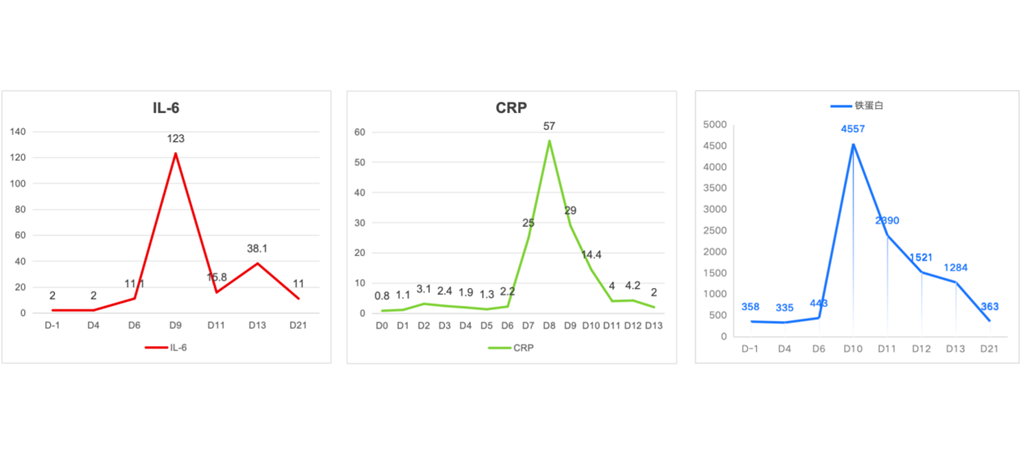
Changes of inflammatory factors in patients
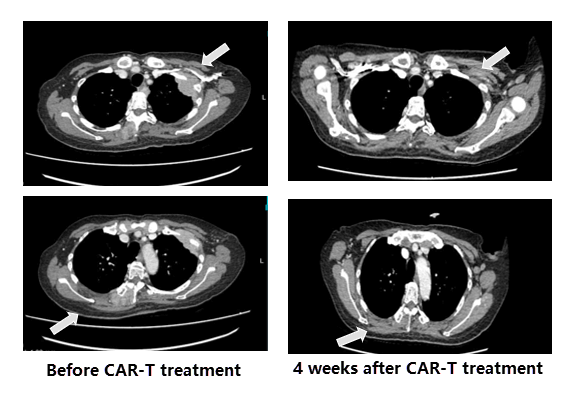
Comparison of CT images before and after CAR-T treatment
Efficacy assessment
On Day21 (D21) after CAR-T reinfusion, the patient’s lumbar and back symptoms were significantly improved, blood/urine M protein was negative, hematuria IFE was negative, and bone marrow flow showed no clonal plasma cells, suggesting hematological remission.
On day28 (D28) after CAR-T reinfusion, CT showed that 2 extramedullary masses had resolved.
About 3 months after CAR-T reinfusion, the patient’s blood/urine M protein was negative, hematuria IFE was negative, no clonal plasma cells were found in bone marrow flow, PET-CT showed that extramedullary masses subsided without hypermetabolic lesions. The effect was CR.
At present, the daily life of the patient has returned to normal. Every time he comes to the hospital for reexamination, he will “take the opportunity” to enjoy Shanghai cuisine.
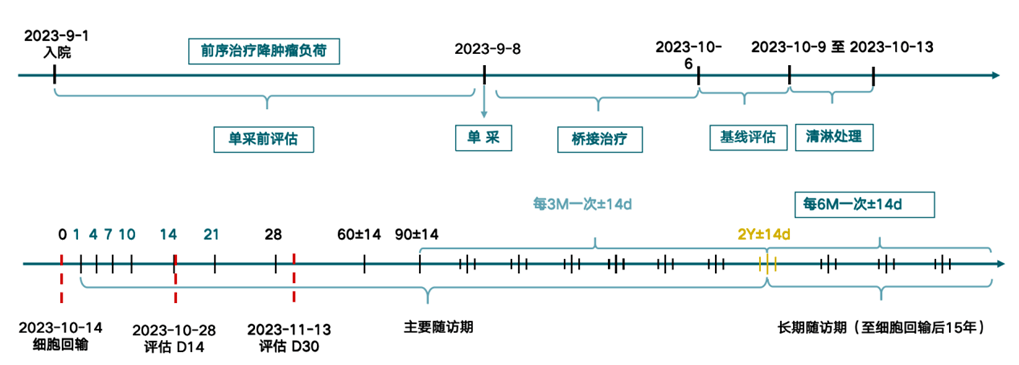
Timeline Review of Equecabtagene (BCMA CAR-T) Treatment
Professor Li Ping’s Experience in Medication
This patient is IgA κ type R/R MM and has undergone multiple lines of therapy. In bridging therapy, the disease progressed despite radiation therapy, suggesting that the disease was very dangerous. After CAR-T reinfusion therapy, hematological CR was achieved in 22 days, and pain and anemia symptoms were significantly improved.
In addition, this patient had a very high tumor burden prior to CAR-T treatment, with bone marrow tumor cells reaching 80%. However, a grade II CRS was only seen at D5, which improved with symptomatic management, and no ICANS occurred, with a very good safety profile, which was also unexpected.
Therefore, through CAR-T treatment for this patient, we also deeply realized the unique advantages of Equecabtagene Autoleucel in the treatment of R/R MM with high potency and low toxicity. It is expected that the patient will maintain a long-term complete response at follow-up.
Professor Liang Aibin comments
As the number of relapses increases and the number of treatment lines increases, the response to treatment becomes worse and the duration of remission becomes shorter. CAR-T therapy is a landmark technology of cellular immunotherapy.
This patient with MM has multiple high-risk genetic factors, has been resistant to protease inhibitors (PI), immunomodulatory drugs (IMiDs) and CD38 monoclonal antibody, and has a very poor prognosis.
CR was achieved after 22 days of self-CAR-T treatment after Equecabtagene Autoleucel injection, confirming the superior efficacy of CAR-T treatment in R/R MM with a single injection. It should be emphasized that close monitoring and correct handling of CRS and ICANS during CAR-T treatment are also key to patient safety and treatment success.

The patient’s family sincerely thanks the medical team of Shanghai Tongji Hospital
References
1. NCCN Guidelines Version 1.2024 Multiple Myeloma.
2. Hematologist Society of Chinese Medical Doctor Association, et al. [J]. Chinese Journal of Internal Medicine, 2022, 61(5): 480-487.
Related Article


