Table of Contents
Prof. Qiu Lugui, Prof. Zou Dehui and Prof. Xu Yan: Equecabtagene combined with ASCT helps ultra-high-risk MM patients bloom flowers of hope.
Multiple myeloma (MM) is an incurable neoplastic disease. Due to the high heterogeneity of the disease, high-risk patients have limited benefit from proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs) and autologous hematopoietic stem cell transplantation (ASCT).
In particular, MM patients with ultra-high cytogenetic risk (≥2 high-risk cytogenetic abnormalities) have a worse prognosis[1-2]. Improving the prognosis of such patients is one of the important challenges of MM treatment at present, and chimeric antigen receptor T cell (CAR-T) therapy is expected to bring new breakthroughs to the treatment dilemma of these ultra-high-risk MM patients.
Professor Xu Yan of Hematology Hospital of Chinese Academy of Medical Sciencesis invited to share the treatment experience of Equecabtagene Autoleucel in treating ultra-high risk MM patients with cytogenetics. Professor Qiu Lugui and Professor Zou Dehui of Hematology Hospital of Chinese Academy of Medical Sciences comment on the case.
Patient’s general conditions
A 67-year-old woman was diagnosed with MM for more than a year. In July 2022, the patient went to other places for treatment due to fatigue, chest distress and shortness of breath after exercise, and scattered ecchymosis all over the body. Bone marrow morphology examination showed obvious increase of plasma cells, accounting for 21%. MM was considered, and then admitted to our hospital for treatment. No previous underlying disease history, ECOG score 1.
Patient baseline examination
Blood routine: WBC 3.20×109/L, RBC 4.00×1012/L, HGB 129g/L, PLT 118×109/L.
Hemochemistry: ALB 41.30g/L, ALT 20.20U/L, AST 14.20U/L, Cr 63.30umol/L, ß2-MG 9.38mg/L, LDH 169.9U/L.
Blood M protein identification: immunoglobulin A 63.10g/L↑, M protein 42.8g/L, free LAM light chain 1362.50mg/L↑; blood immunofixation electrophoresis showed a monoclonal IgA λ component in γ region.
Urine M protein identification: urine M protein quantitative 986.96 mg/24h, urine immunofixation electrophoresis in the γ region can be seen a monoclonal λ component.
Bone marrow smear: plasma cell ratio 28%.
Bone marrow biopsy: abnormal plasma cells 30%.
Whole body MRI revealed diffuse + nodular multiple myeloma lesions throughout the skeleton.
Gene mutation detection: RB-1 gene deletion positive, P53 deletion positive, CKS1B gene amplification positive.
FISH: 13q- (83%), 1q21+ (92%), 17p- (54%).
Clinical diagnosis
MM-IgAλ type, DS stage ⅢA, ISS stage Ⅲ, R-ISS stage Ⅲ, R2 ISS stage IV (with 13q-, 17p-, 1q21 +), belongs to cytogenetics super high risk.
Treatment Process
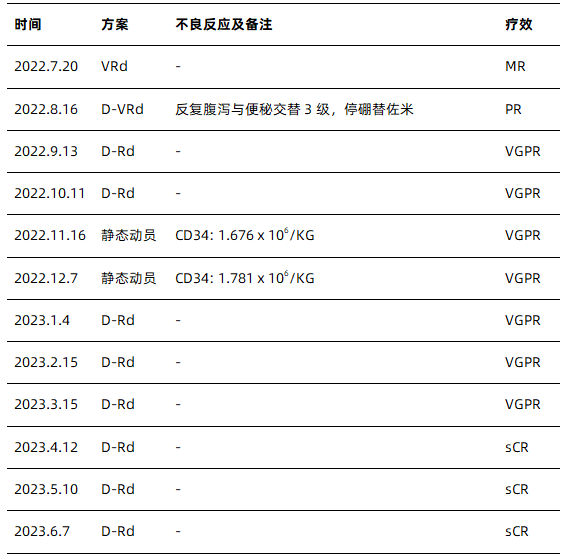
*V: bortezomib; R: lenalidomide; d: dexamethasone; D: daratumumab; PR: partial response; VGPR: very good partial response; sCR: strict complete response;
Because patients are at high risk for double-hit ultra-high-risk MM, the risk of early recurrence and progression is extremely high, and the median survival expected for such patients is less than 2 years [3]. At present, he has received 10 courses of induction therapy, and has achieved sCR, but the minimal residual disease (MRD) test is still positive, and MRD is closely related to the patient’s prognosis. Obtaining MRD negative may help overcome the poor prognosis of high-risk cytogenetics [4].
FUMANBA-1 study [5] showed that Equecabtagene can achieve deep response in the treatment of multi-line relapsed/refractory (R/R) MM, with an overall response rate of more than 95%, 95.0% of subjects achieving MRD negative, among which all CR/sCR subjects achieved MRD negative, with good safety. The clinical study of Equecabtagene in second-line R/R MM is being conducted subsequently. Other studies of BCMA CAR-T have been reported in the early and even first-line treatment of MM [6].
Our center is also exploring ASCT combined with CAR-T for first-line treatment of ultra-high-risk MM. At present, 8 patients have been reinfused, and 6 patients who can be evaluated for MRD have obtained MRD negative.
Based on the above background, the patient and his family decided to receive ASCT+CAR-T cells (Equecabtagene) after consultation, in order to further eliminate tumor cells, improve the depth of response, improve the patient’s own anti-tumor immune function, and finally prolong the patient’s survival.
Assessment before lymph collection: no active infection in recent 7 days; stable vital signs; ECOG 1; viral infection (-); blood routine: Hb 127g/L, ALC 0.84×109 /L, PLT 125×109 /L; normal coagulation function;
Bridging therapy: In July 2023, after lymphocyte collection, bridging therapy with D-Rd protocol was given.
Pre-lymphocyte-depleting chemotherapy assessment: no infection, normal vital signs, peripheral oxygen saturation 100%, normal liver and kidney function, cardiac ejection fraction 63%, normal CRP, ferritin 60.20 ng/ml (reference value: 11.0- 306.8 ng/ml).
Pretreatment: High-dose melphalan (HD-Mel regimen).
Pre-infusion evaluation of Equecabtagene: ECOG score 0; no infection manifestations; normal vital signs; peripheral oxygen saturation 100%; no arrhythmia, hypotension and heart failure manifestations; no new or other non-hematological organ dysfunction; no corticosteroids used within 3 days.
ASCT was performed on the fourth dayafter pretreatment, and Equecabtagene was infused on the sixth day.
Safety monitoring: fever occurred on the third day after CAR-T reinfusion, with the highest temperature of 38.8℃, normal blood pressure and oxygen saturation. Cytokine release syndrome (CRS) grade 1 was considered. As the patient had severe neutropenia, concomitant infection could not be excluded, so broad-spectrum antibiotics were given for anti-infection and symptomatic antipyretic treatment.
Because there was no obvious sign of body temperature control, IL-6 showed a trend of increase (see Figure 1 for changes of inflammatory factors in patients). Methylprednisolone 40 mg was applied on the 4th day after CAR-T reinfusion, IL-6 level decreased significantly, and body temperature was gradually controlled.
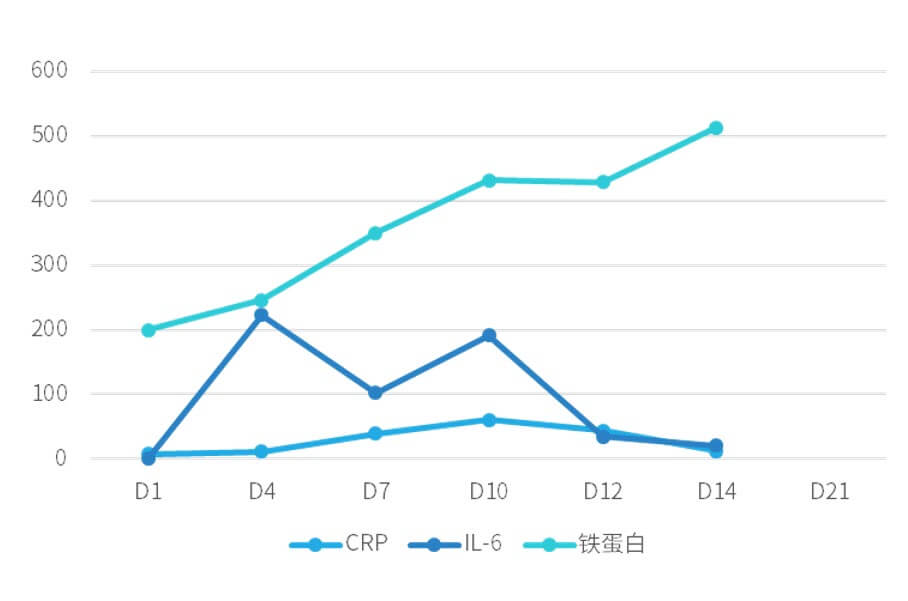
Figure 1. Changes of inflammatory factors in patients
Evaluation 14 days after Equecabtagene reinfusion: CRS recovered after treatment, no immune effector cell-related neurotoxicity syndrome (ICANS) occurred, infection was controlled, severe cytopenia recovered, blood biochemical tests showed no obvious abnormalities, and the patient was discharged after evaluation by medical institutions.
Efficacy assessment one month after discharge: hematuria protein 0; bone marrow biopsy and bone marrow smear: plasma cells 0%; flow detection minimal residual disease (MRD) assessment negative (10-5). The patient’s efficacy assessment was sCR with MRD negative (see Figure 2 for specific efficacy assessment results).
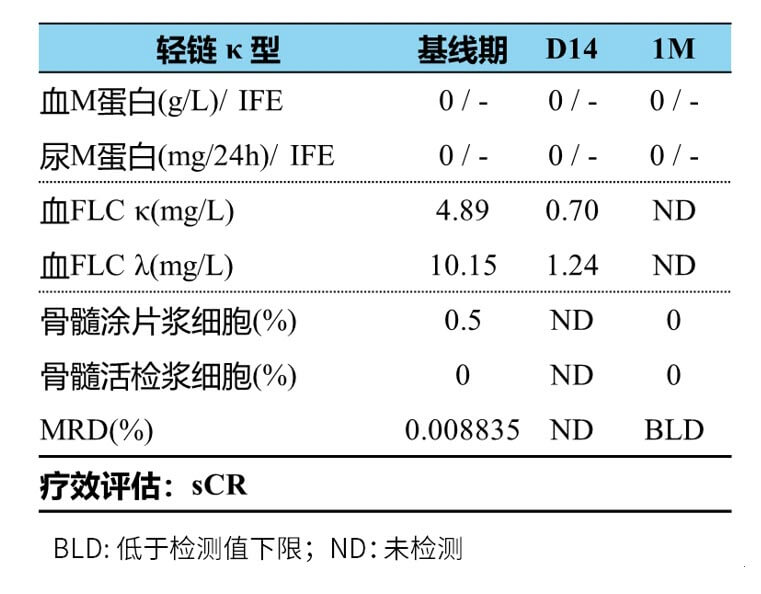
Figure 2. Results of efficacy evaluation
Professor Xu Yan’s Experience in Medication
This patient is a cytogenetic ultra-high risk MM patient who failed to achieve deep response (MRD negative) after treatment with VRd, D-VRd and D-Rd regimens within a short period of one year. The survival time of such patients is expected to be less than 2 years[3].
To improve the survival of such patients, research centers are constantly exploring, and CAR-T treatment is the promising choice for such patients. After full communication with the patient and his family, ASCT combined with CAR-T (Equecabtagene) was given in August 2023. Only grade 1 CRS occurred after the reinfusion of Equecabtagene, which was improved after symptomatic treatment. There was no ICANS and no delay in hematopoietic reconstitution.
The patient was discharged smoothly 14 days after reinfusion. The efficacy was sCR and MRD was negative 1 month after reinfusion. It is expected that the patient will remain MRD negative at follow-up and eventually translate to long-term disease-free survival.
Comments by Professors
Professor Zou Dehui
ASCT plays an important role in MM patients eligible for transplantation, but recurrence is still difficult to avoid, especially in patients with cytogenetic ultra-high risk, and survival is expected to be less than 2 years[3].
For such patients, how to achieve sustained and deep remission remains unresolved and needs to be explored. In recent years, BCMA CAR-T has made great progress in the treatment of R/R MM. Therefore, CAR-T in combination with ASCT is a very promising and desirable treatment strategy for such patients.
Professor Qiu Lugui
This patient is a 67-year-old patient with cytogenetics at ultra-high risk of MM treated with third-line therapy. Although his general condition is good, he did not achieve deep remission after induction therapy.
According to the comprehensive evaluation of the disease condition, CAR-T (Equecabtagene) combined with ASCT was selected for treatment. MRD turned negative 1 month after CAR-T reinfusion, and only grade 1 CRS occurred during the treatment period.
Adverse reactions were mild and improved after symptomatic treatment. This case fully demonstrates that Equecabtagene combined with ASCT can achieve deep response and good safety in patients with cytogenetic ultra-high risk MM, bringing hope for long-term survival to patients, and looking forward to follow-up results.
In the future, it is hoped that innovative therapies and protocols such as CAR-T therapy will bring survival benefits to more patients with cytogenetics and ultra-high risk MM, and bring hope to such patients.
References
[1] Cinnie Yentia Soekojo, MD, et al.Blood (2020) 136 (Supplement 1): 51–52.
[2] Pasvolsky O, et al. Transplant Cell Ther. 2023;S2666-6367(23)01513-0.
[3] Liu Shan, et al. Chinese Journal of Oncology, 2021, 43 (11): 1209-1214.
[4] Paiva B, et al. J Clin Oncol. 2020;38(8):784-792.
[5] Chunrui Li, et al. 2023 ASCO. Abstract 8025.
[6] Shi X, et al. Am J Hematol. 2022;97(5):537-547.
Related Article


