Table of Contents
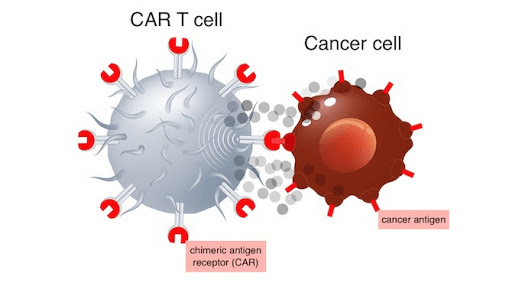
Immunotherapy is the fourth major cancer treatment method following surgery, radiotherapy, and chemotherapy 1. Cancer immunotherapy represented by Chimeric Antigen Receptor (CAR)-T cell therapy was named the top of the “Top 10 Scientific Breakthroughs” by Science in 2013.

It is a method that uses patient’s own immune T cells to fight cancer, where these cells are genetically modified in the laboratory to more effectively target and destroy cancer cells.
As a significant scientific breakthrough, CAR-T cell therapy brings new hope to patients with multiple myeloma (MM). Currently, there are three CAR-T cell therapies approved for the treatment of MM internationally: FUCASO® (Equecabtagene Autoleucel, Eque-cel), ABECMA® (Idecabtagene Vicleucel, ide-cel), and CARVYKTI® (ciltacabtagene autoleucel, cilta-cel).
Eque-cel
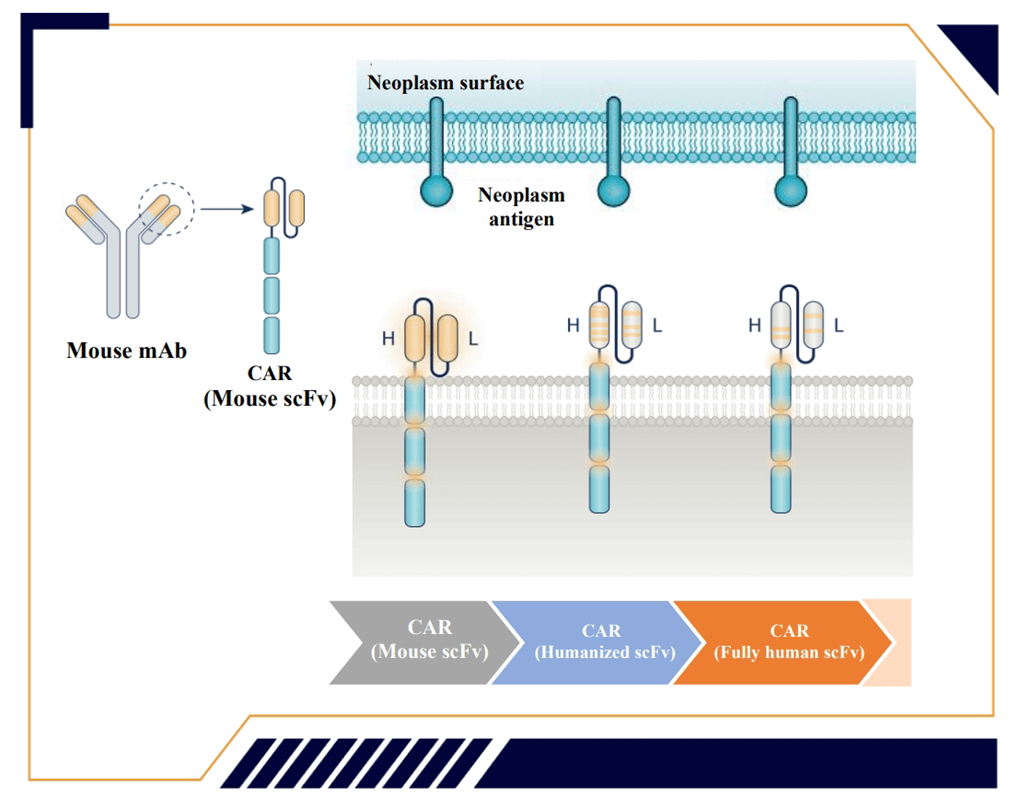
Eque-cel, developed by IASO Biotechnology, was approved by the China National Medical Products Administration (NMPA) in 2023 for the treatment of patients with relapsed refractory multiple myeloma (RRMM) and is the world’s first approved fully human BCMA-targeting CAR-T cell therapy for RRMM.
It has also been granted Orphan Drug Designation (ODD), Regenerative Medicine Advanced Therapy (RMAT), and Fast Track (FT) status by the FDA, as well as approval for Investigational New Drug (IND) application in the United States. The treatment cost is within 200,000 US dollars per injection.
As a fully human CAR-T cell therapy, Eque-cel is characterized by low immunogenicity, significant expansion, and long in vivo persistence. The incidence of anti-drug antibodies (ADA) at 3 months is 2.6%, with over 50% of patients having in vivo persistence for more than 1 year, and over 40% of patients having in vivo persistence for more than 2 years, with a median persistence of 419 days 2,3.
Results from the FUMANBA-1 study indicate that, with a median follow-up of 13.8 months, the overall response rate (ORR) was 98.9% in 103 patients with RRMM who had no prior CAR-T treatment history and received Eque-cel treatment, with 82.4% of patients achieving complete response or better (≥ CR), 97.8% achieving minimal residual disease (MRD) negativity, 80.8% achieving 12-month sustained MRD negativity, and 85.5% achieving 12-month pregression-free survival (PFS) 2,4-6. One patient experienced ≥ Grade 3 cytokine release syndrome (CRS), and no patients experienced ≥ Grade 3 immune effector cell-associated neurotoxicity syndrome (ICANS).
Ide-cel
Ide-cel was co-developed by Bristol-Myers Squibb and Bluebird Bio. It was approved by the FDA in 2021 for the treatment of RRMM patients, becoming the world’s first BCMA CAR-T cell therapy that received regulatory approval. Previously, the therapy had been granted Breakthrough Therapy Designation by the FDA and had received PRIME designation from the European Medicines Agency. The treatment cost is approximately 524,833 US dollars per injection.
Ide-cel is a murine-derived CAR-T product, and animal-derived products may have potential immunogenicity, be prone to develop immune rejection by the human body, and increase the risk of unintended binding with human tissues or cells. The incidence of ADA at 3 months for ide-cel is 21%, with 59% of patients achieving 6-month in vivo persistence and 36% achieving 12-month in vivo persistence.
Results from the KarMMa study showed that with a median follow-up of 13.3 months, the ORR was 73% in 128 patients with previously untreated RRMM following bridging therapy who were treated with ide-cel, with 33% of patients reaching ≥ CR and 26% achieving MRD negativity, and a median PFS of 8.8 months. After ide-cel treatment, 5% of patients experienced ≥ Grade 3 CRS, and 3% experienced Grade 3 neurotoxicity 8.
Cilta-cel
Cilta-cel, developed by Legend Biotech (Figure 5), was approved by the FDA in 2022 for the treatment of RRMM patients, making it the second BCMA CAR-T cell product and also the first Chinese cell therapy product that received FDA approval. The treatment cost is approximately 504,344 US dollars per injection.
Cilta-cel is an alpaca/camel-derived CAR-T product, which also has potential immunogenicity and risks of immune rejection associated with animal-derived products. The incidence of ADA at 3 months for cilta-cel is 31.3%, with a median persistence of 57 days.
Results from the CARTIFAN-1 study showed that with a median follow-up of 18 months, patients with previously untreated RRMM who were treated with cilta-cel achieved an ORR of 89.6%, with 77.1% reaching ≥ CR 9, 53.1% achieving a 12-month sustained MRD negativity 10 and 77% achieving 1-year PFS 11. The incidence of ≥ Grade 3 CRS was 35.4%, and the incidence of ≥ Grade 3 ICANS was 5.2%.
The CARTITUDE-1 study showed that life-threatening Parkinson’s disease can occur with the clinical use of cilta-cel (incidence of 4.1%). Therefore, the FDA black box warning indicates that Parkinson’s disease, Guillain-Barré syndrome, and related life-threatening complications have occurred after treatment with cilta-cel 12.
Final Words
Immunotherapy, as a novel and cutting-edge treatment approach, has milestone significance in the development of MM treatment. CAR-T cell therapy is mainly led by China and the United States, with China being particularly prominent, as the number of clinical trials has jumped to the highest in the world. Eque-cel, as the first “Made in China” fully human CAR-T cell product, has broad prospects and excellent clinical performance, and is expected to bring new hope to MM patients.
References:
1. McCune JS. Clin Pharmacol Ther. 2018;103(4):540-544.
2. 2023 ASCO, abstract 8025
3. Blood. 2021;137(21):2890-2901.
4. 2023 IMS. P-290.
5. 2023 ASH. Oral 761.
6. 2023 ASH. Poster 4854.
7. Rui Cui, et al. Chinese Journal of Hematology, 2021, 42(6) : 502-507.
8. Munshi NC, et al. N Engl J Med. 2021 Feb 25;384(8):705-716.
9. Mi JQ, et al. J Clin Oncol. 2023 Feb 20;41(6):1275-1284.
10. 2023 ASCO. Abstract 8009.
11. Lancet. 2021 Jul 24;398(10297):314-324.
12. CARVYKTI FDA Package Insert.-Feb 2023
13. https://www.drugs.com/price-guide/abecma
14. https://www.drugs.com/price-guide/carvykti


