Table of Contents
EBMT 2024 1st day┃ First Comparison of the Efficacy of Four CAR-Ts for RRMM in the China and U.S: MAIC Analysis Results Released
The 50th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT) was held today in the UK, where a study, “Match-Adjusted Indirect Comparative Analyses of Effectiveness (MAIC)1 of BCMA CAR-T Targeted Drugs in the RRMM,” revealed for the first time indirect comparisons of the effectiveness of four BCMA CAR-T drugs, including overall remission rates (ORR), complete remission (CR) rates, and minor residual disease (MRD) negative rates.
Analysis and Results
The study results showed that in terms of ORR, the world’s first fully human CAR-T, Equecabtagene, which was approved and marketed in China in 2023, showed a trend to outperform Zevorcabtagene and Ciltacabtagene and ide-cel, which are marketed in the U.S.; and in terms of CR rate, Equecabtagene is significantly superior to Zevorcabtagene.
To date four BCMA CAR-Ts have been approved and marketed globally for the treatment of relapsed or refractory multiple myeloma (RRMM) in adults, and there are no head-to-head studies comparing the efficacy of several BCMA CAR-Ts.
The current study, which was adopted by the EBMT Congress, used a matched-adjusted indirect comparison (MAIC) approach to compare the efficacy of the registry-based clinical studies of Equecabtagene Autoleucel(equ-cel), Ciltacabtagene Autoleucel (cilta-cel), Zevorcabtagene Autoleucel(zevor-cel), and Ide-cel (Idecabtagene autoleucel) in a Comparison.
The methodology compares efficacy in matched baseline balanced study populations by weighting individual case data from pharmacological interventions to match pooled data from control interventions.
For this MAIC analysis, the primary data for Equecabtagene were derived from the Registered Clinical Study FUMANBA-1 Phase 1Ib/II, an open-label, single-arm, multicentre study designed to evaluate the efficacy and safety of the world’s first fully human chimeric antigen receptor (CAR)-T cell Equecabtagene Autoleucel (R&D No. CT103A, equ-cel) in a study of efficacy and safety in patients with relapsed refractory multiple myeloma (RRMM) who had received 3 or more prior lines of therapy for a short period of time.
With a median follow-up time of 18.07 months, high depth and persistence of remission was observed in 103 evaluable patients, and in subjects with no prior history of CAR-T therapy, an ORR of 98.9 percent , sCR/CR rate of 82.4% and MRD-negative rate of 97.8%, with a median time to reach MRD-negativity of only 15 days; 12-month progression-free survival (PFS) rate of 85.5%, with MRD-negativity persisting for more than 12 months in 81.7% of the patients3.
| FUMANBA-1 (Equecabtagene) v.s CARTIFAN (Ciltacabtagene) |
FUMANBA-1 (Equecabtagene) v.s LUMMICAR-1 (Zevorcabtagene) |
FUMANBA-1 (Equecabtagene) v.s KARMMA (ide-cel) |
FUMANBA-1 (Equecabtagene) v.s CARTITUDE1 (Ciltacabtagene) |
|
|---|---|---|---|---|
| MAIC (ESS*=75) | MAIC (ESS=49.4) | MAIC (ESS=10.7) | MAIC (ESS=10.7) | |
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| ORR | 5.65 (0.94,33.9) | 5.17 (0.69,38.82) | 11.57 (0, inf) | 0.19 (0, inf) |
| ≥CR rates | 1.15 (0.49,2.71) | 6.08 (2.88,12.83) | 3.20 (0, inf) | 0.29 (0, inf) |
| MRD-negtive rates | 0.8(0.08,8.36) | / | 28.59 (0, inf) | 0.19 (0, inf) |
| MAIC-weighted With CARTIFAN for Equecabtagene | CARTIFAN | MAIC-weighted With LUMMICAR-1 for Equecabtagene | LUMMICAR-1 | MAIC-weighted With KARMMA for Equecabtagene | KARMMA | MAIC-weighted With CARTITUDE1 for Equecabtagene | CARTITUDE1 | |
|---|---|---|---|---|---|---|---|---|
| 12m PFS rates | 82.3% | 77% | 93.2% | / (9m PFS rate 84.6%) | 79.7% | / | 94.2% | 75%# |
| 12m MRD Persistent negative rates | 77.8% | / | 76.4% | / | 84% | 19.5% | 100% | 53.1% |
The MAIC analysis, which weighted and matched individual case data from the FUMANBA-1 study, showed that in the comparison of ORR (overall remission rate), Equecabtagene was 5.65 times higher than Ciltacabtagene (CARTIFAN study), with a superiority ratio (OR value) of 5.65 and a 95% confidence interval (0.94, 33.9), and that in the comparison of CR rate (complete remission), Equecabtagene was 6.08 times higher than Zevorcabtagene (LUMMICAR study) by a factor of 6.08, with a statistically significant difference in improvement advantage, with an advantage ratio (OR value) of 6.08 and a 95% confidence interval (2.88, 12.83).
In the comparison of long-term efficacy: the 12-month PFS rate of 94.2% for Equecabtagene was higher than that of 75% for Ciltacabtagene (CARTITUDE-1 study); and the 12-month sustained MRD negativity rate of 100% for Equecabtagene was higher than that of 53.1% for Ciltacabtagene (CARTITUDE-1 study). The comparison of the two long-term efficacy indicators showed that the Chinese-launched BCMA CAR-T drug, Equecabtagene, has a trend of superior long-term efficacy compared to the US-approved Ciltacabtagene.
Investigators’ Insights
The data reported at this congress further demonstrated that Equecabtagene is a more promising treatment option for patients with relapsed refractory multiple myeloma, showing a trend toward superior short- and long-term efficacy improvement under relatively balanced conditions at baseline after matched adjustment for prognostic factors with controlled studies.
The principal investigators of this clinical study, Professor Qiu Lugui from the Hospital of Haematology, Chinese Academy of Medical Sciences, and Professor Li Chunrui from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, said, “BCMA CAR-T has shown breakthrough efficacy compared to conventional treatments, and the current MAIC analysis, which compares the efficacy of different CAR-Ts under the condition of balanced baseline, showed that among the In a comparison of the prognostically important endpoints for MM patients, such as objective remission rate, CR rate, MRD-negative rate, and sustained-negative rate, Equecabtagene showed superior short- and long-term efficacy among the four BCMA CAR-T drugs, and superior long-term efficacy when compared to similar drugs approved in the United States.
Different CAR-T drugs have different CAR structures and R&D ideas. The CAR structure of Equecabtagene is selected from a large number of fully human alternative antibodies, and the light and heavy chain fully human antibody epitopes are tightly bound to the BCMA antigen to enhance the tumour-killing ability (Figure 1), and the low immunogenicity of Equecabtagene can prolong the time of Equecabtagene’s survival in the patient’s body; moreover, Equecabtagene’s dissociation constants (Kd) are very close to those of the human body.
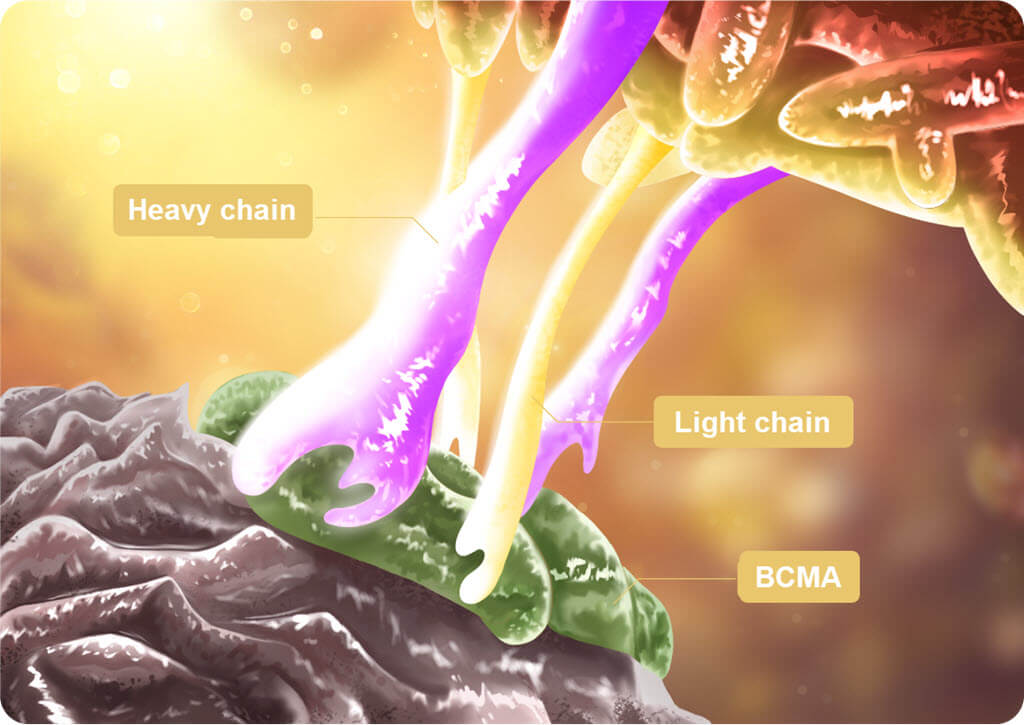
Figure 1
Natural T-cell dissociation kinetics2 (Figure 2): fast dissociation is conducive to the efficient activation, killing, and proliferation of CAR-T cells in vivo, which, while enhancing the tumour-killing efficiency and the depth of remission, is able to reduce the self-depletion of Equecabtagene, which is conducive to the persistent continuation of the patient’s body, the long-term performance of the surveillance role, and the reduction of the risk of relapse.“
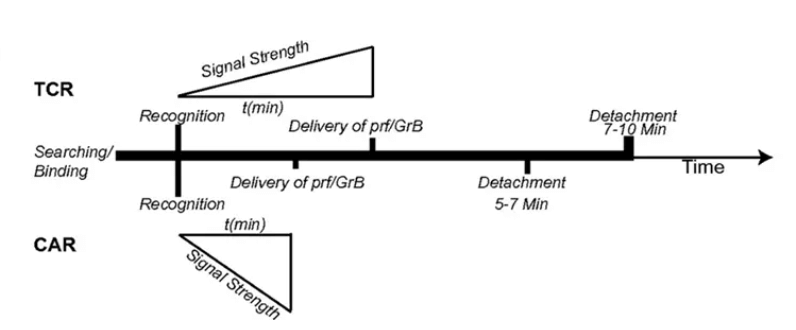
Figure 2
In registered clinical trials in the Chinese population, Equecabtagene also showed an excellent safety profile: only 1% grade ≥3 CRS; no grade ≥3 ICANS; no motor/cognitive deficits, no Parkinson’s disease, and no secondary tumours were detected. The 10-month launch in China has already accumulated more clinical application experience, which helps to deeply understand the efficacy and safety of BCMA CAR-T therapy in the Chinese population.
Final Words
Currently, BCMA CAR-T drugs are mainly approved for the treatment of relapsed or refractory multiple myeloma, and its potential application in more indications, such as myasthenia gravis and other serious autoimmune diseases, has also made encouraging progress in China; China’s independently researched, developed and fully produced BCMA CAR-T drugs have excellent efficacy and safety, and have a promising future for global application, which will bring hope of a cure to more patients in China and internationally.
References
[1] 2024 EBMT, Abstract A092.
[2] Davenport, A. J., et al.” Proceedings of the National Academy of Sciences 115, no. 9: E2068–76.
[3] 2023 IMS. P-290.
Related Article


