Table of Contents
Functional High-Risk Multiple Myeloma Insights
As a highly heterogeneous group of hematologic malignancies, the use of proteasome inhibitors (PIs), immunomodulators (IMiDs), and monoclonal antibodies has improved patient prognosis and survival. In recent years, some investigators have included patients with poor induction therapy response and/or early progression “Functional high-risk Multiple Myeloma”. This group of patients has been found to have a poor survival prognosis, and there is an urgent need for new drugs and new therapies to bring clinical benefits.
As an innovative therapy for Multiple Myeloma, BCMA CAR-T cell therapy has been fully proven to have good efficacy and safety, and its application in patients with functional high-risk Multiple Myeloma is also in full swing.
This article will focus on the definition and characteristics of functional high-risk Multiple Myeloma and the application of BCMA CAR-T in such patients, and Professor Lu Jin of Peking University People’s Hospital will interpret this hot topic for the benefit of readers.
Prognosis of Functional High-Risk Multiple Myeloma
Risk stratification is essential for the management of Multiple Myeloma. Currently, the risk stratification approach is based on genetic information from FISH as well as clinical information from the R-ISS staging system. Gene expression features such as EMC92 and GEP70, as well as clinical features such as extramedullary plasmacytoma, circulating tumor cells, and renal failure are also associated with high-risk diseases, but they are still being studied and have not been included in clinical trial standards and clinical practice1.
In recent years, the concept of “functional high risk” (FHR), which is different from standard risk (SR) and genomic high risk (GHR), has been widely concerned and studied.
The Australian and New Zealand Myeloma and Related Disease Registry (MRDR) analysed available data from 1,320 newly diagnosed Multiple Myeloma (NDMM) patients in the real world with the aim of determining disease characteristics in patients with FHR Multiple Myeloma.
Study First-line (1L) sub-optimal responders (SORs) were defined as those who did not respond well to induction therapy (optimal response was minimal response [MR] or stable disease [SD]), and early progressors (EP) who experienced early disease progression within 12 months of induction therapy, regardless of whether progression occurred at or after treatment completion.
Median follow-up of 23 months,152 (11.5%) patients with NDMM did not respond well to induction therapy, and 118 (8.9%) patients with NDMM developed early disease progression within 12 months of induction therapy. In addition, the prognosis of patients with Multiple Myeloma who are classified as FHR in the above two categories is significantly worse2.
Over the past few decades, with the introduction of drugs such as PIs, IMiDs, and monoclonal antibodies, as well as the combination with autologous hematopoietic stem cell transplantation (ASCT), the median overall survival (OS) of Multiple Myeloma patients has increased from 3 years to 8 years, but 20% of patients still survive for only about 3 years. Improving the prognosis of these high-risk patients is one of the important treatment challenges for Multiple Myeloma1.
The above MRDR real-world study found that the prognostic factors associated with SOR included age > 70 years at diagnosis (P<0.001) and the absence of bortezomib in the 1L regimen (P<0.001).
The median OS of SOR was similar to that of patients with ≥ partial response (PR) (R), but there were more patients with early death in SOR, and compared with R.
The 12-month OS rate was 85% vs. 96%, the 24-month OS rate was 73% vs. 88%, and the 36-month OS rate was 61% vs. 79%, respectively (P=0.001). It can be seen that 40% of SOR patients die within 3 years of diagnosis2.
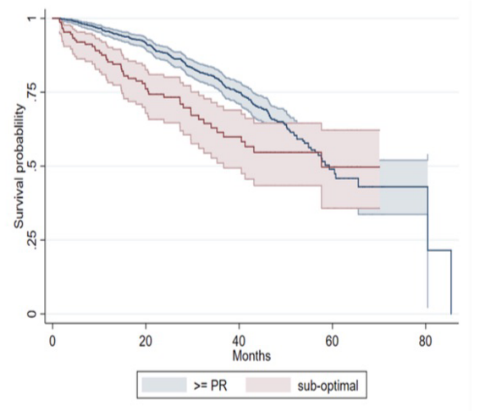
Figure 1. OS in SOR versus R patients.
EP is highly associated with a high disease burden at diagnosis, including ISS, R-ISS, late ECOG stage, hypercalcemia according to CRAB criteria, renal insufficiency, and anemia, and the likelihood of high refractory disease at first relapse. Compared with non-EP, the median OS of EP patients was significantly shortened, which was 20.2 months vs. 60.7 months (P<0.001)2.
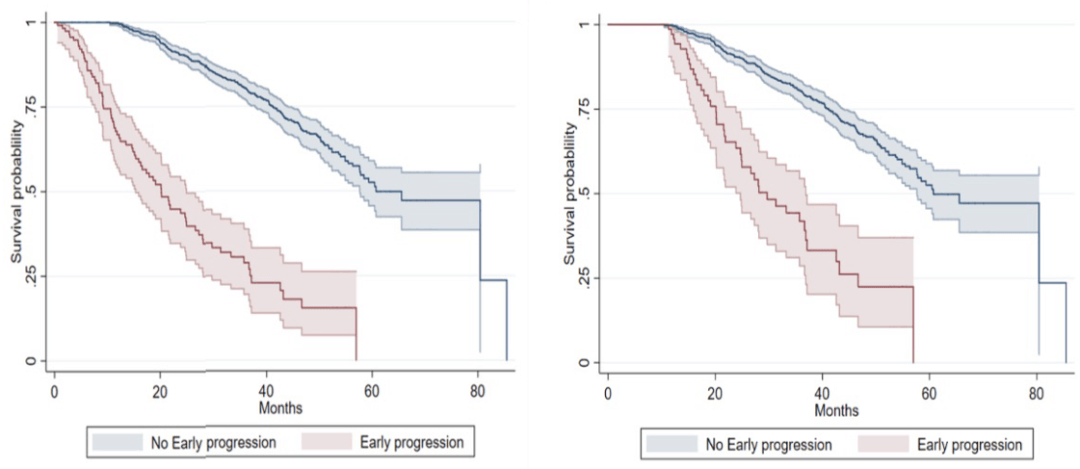
Figure 2. OS in patients with EP versus non-EP measured at diagnostic measurements (left) and 12 months (right).
On the basis of this study, the criteria for defining FHR patients were evaluated more precisely. Of the 512 evaluable patients in the study, there was a significant difference between the SR group (who did not meet any criteria for GHR or FHR; n = 345) and the GHR group (t [4;14], t[14;16], complete loss of functional TP53, 1q21 amplification, or ISS stage III; n=106).
Compared with patients in the FHR group (no GHR markers but refractory to induction therapy or early recurrence at 12 months) had the worst survival (Fig. 3). Patients with FHR typically do not have known high-risk features, but have increased mutations in the IL-6/JAK/STAT3 pathway and gene expression profiles associated with aberrant mitotic and DNA loss responses1.
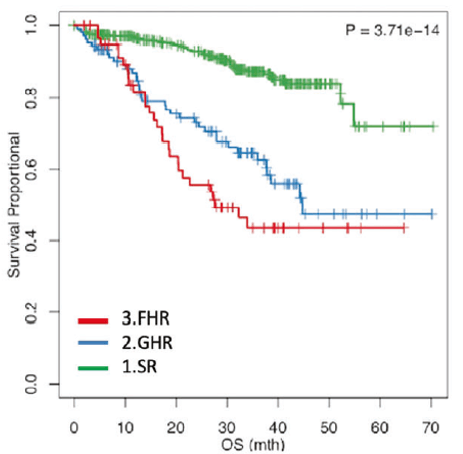
Figure 3. OS in the R, GHR, and FHR groups
The study showed that patients with FHR Multiple Myeloma had a worse prognosis, even in the absence of any high-risk genetic factors. Therefore, there is an urgent need to further accurately identify patients with FHR Multiple Myeloma and explore new drugs and therapies with different mechanisms of action to provide treatment options for these patients, with a view to improving remission rates and prolonging survival for these patients.
BCMA CAR-T for Long-Term Relief in High-Risk Multiple Myeloma
BCMA is a transmembrane protein that is highly expressed on malignant plasma cells and low in normal tissues, and is a good diagnostic marker and therapeutic target for Multiple Myeloma.
BCMA CAR-T cell therapy has emerged as one of the most promising treatment options for Multiple Myeloma, which can be well expanded and sustained in Multiple Myeloma patients through a one-time injection, bringing long-term deep remission and prolonging survival.
For functional high-risk patients with poor prognosis, BCMA CAR-T cell therapy may be one of the treatment options for patients with full efficacy and safety advantages.
A subgroup analysis of a Phase II clinical study evaluating the efficacy and safety of BCMA CAR-T in patients with early recurrence (≤ 12 months after ASCT or ≤12 months after initiation of initial anti-myeloma therapy without ASCT) included 19 patients (16% GHR, 63.2% SR, and 21.1% unknown), 79% of whom had received ASCT. The primary endpoint was minimal residual disease (MRD) negative rate (MRD 10-5)3.
The results of the study showed that with a median follow-up of 17.8 months, the overall response rate (ORR) was 100%, with 100% achieving ≥ very good partial response (VGPR) and 90% achieving ≥ complete response (CR)(Figure 4). The median time to first and best response was 0.95 months and 5.1 months, respectively. Of the evaluable patients with MRD (n=15), 14 (93%) achieved MRD negativity during the study. Median duration of response was not reached, with a 12-month event-free (EFS) rate of 84% and a 12-month progression-free survival (PFS) rate of 90%3.
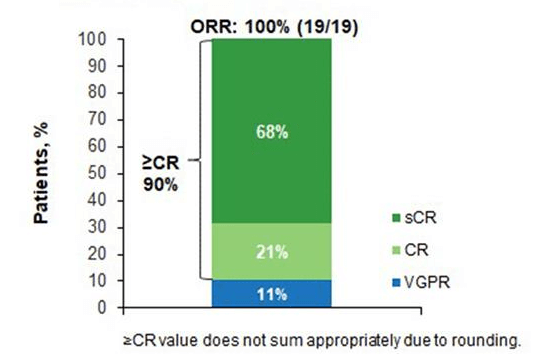
Figure 4. Remission after treatment with BCMA CAR-T cells
In terms of safety, the most common treatment-related adverse events (TEAEs) were hematologic AEs (grade 3/4: neutropenia, 90%; lymphopenia, 42%; thrombocytopenia, 26%; leukopenia, 26%). The median time from BCMA CAR-T infusion to the onset of cytokine release syndrome (CRS) was 8 days, and patients with CRS were in remission after symptomatic management. One patient developed immune effector cell-associated neurotoxicity syndrome (grade 1)3.
In this study, 90% of patients with early recurrence did not progress on the disease at 1 year after BCMA CAR-T infusion. The results of a longer (18-month) follow-up showed that patients with functional high-risk Multiple Myeloma received durable and deepened remission after BCMA CAR-T therapy, and the PFS rate remained unchanged. The results of this study suggest that BCMA CAR-T has a promising future in people with large unmet needs3.
Final Words
Excluding any known high-risk genetic factors that have been used in clinical practice, or other published high-risk characteristics, the prognosis for most FHR Multiple Myeloma remains poor.
In addition, the current definition of a patient with FHR Multiple Myeloma is based on poor response to induction therapy and early disease progression, and there are no clear assays or biomarkers to identify them at the time of diagnosis. In the future, it is of great significance to understand the genomics and biology of FHR Multiple Myeloma patients, more clearly identify FHR patients, and design clinical trials specifically for FHR patients.
The sustained and deep response of BCMA CAR-T cell therapy in patients with early relapsed Multiple Myeloma may indicate that this therapy can be used as a new treatment option for FHR Multiple Myeloma patients to prolong their survival.
As the first BCMA CAR-T cell immunotherapy product in China, Equecabtagene Autoleucel was approved by the National Medical Products Administration (NMPA) of China for the treatment of adult patients with relapsed or refractory Multiple Myeloma (R/R Multiple Myeloma) on June 30, 2023.
It is expected that Equecabtagene Autoleucel can be further explored in patients with FHR Multiple Myeloma, advancing its treatment for RRMultiple Myeloma to the frontline, improving quality of life and survival outcomes in patients with FHR Multiple Myeloma!
References
1. Soekojo CY, et al. Blood Cancer J. 2022 Jan 31; 12(1):24.
2. Andrew Spencer, et al. Blood (2019) 134 (Supplement_1): 269.
3. Niels WCJ Van De Donk, et al. Blood (2022) 140 (Supplement 1): 7536–7537.
Related Article


