Providing Full-Process Support for International Patients Coming to China for CAR-T Cell Therapy
CAR-T Therapy for International Multiple Myeloma Patients
China CAR-T Leading Advantages: Why Choose to Receive Car-T for Multiple Myeloma in China?
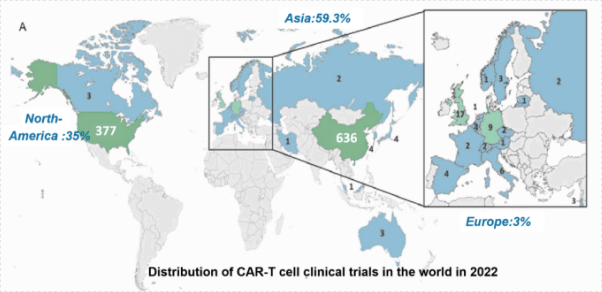 Resource: Cancers 2023,15,1003
Resource: Cancers 2023,15,1003
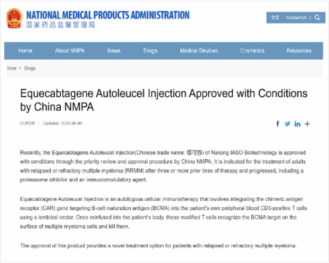
IASO Equecabtagene Autoleucel (BCMA CART) NDA approval
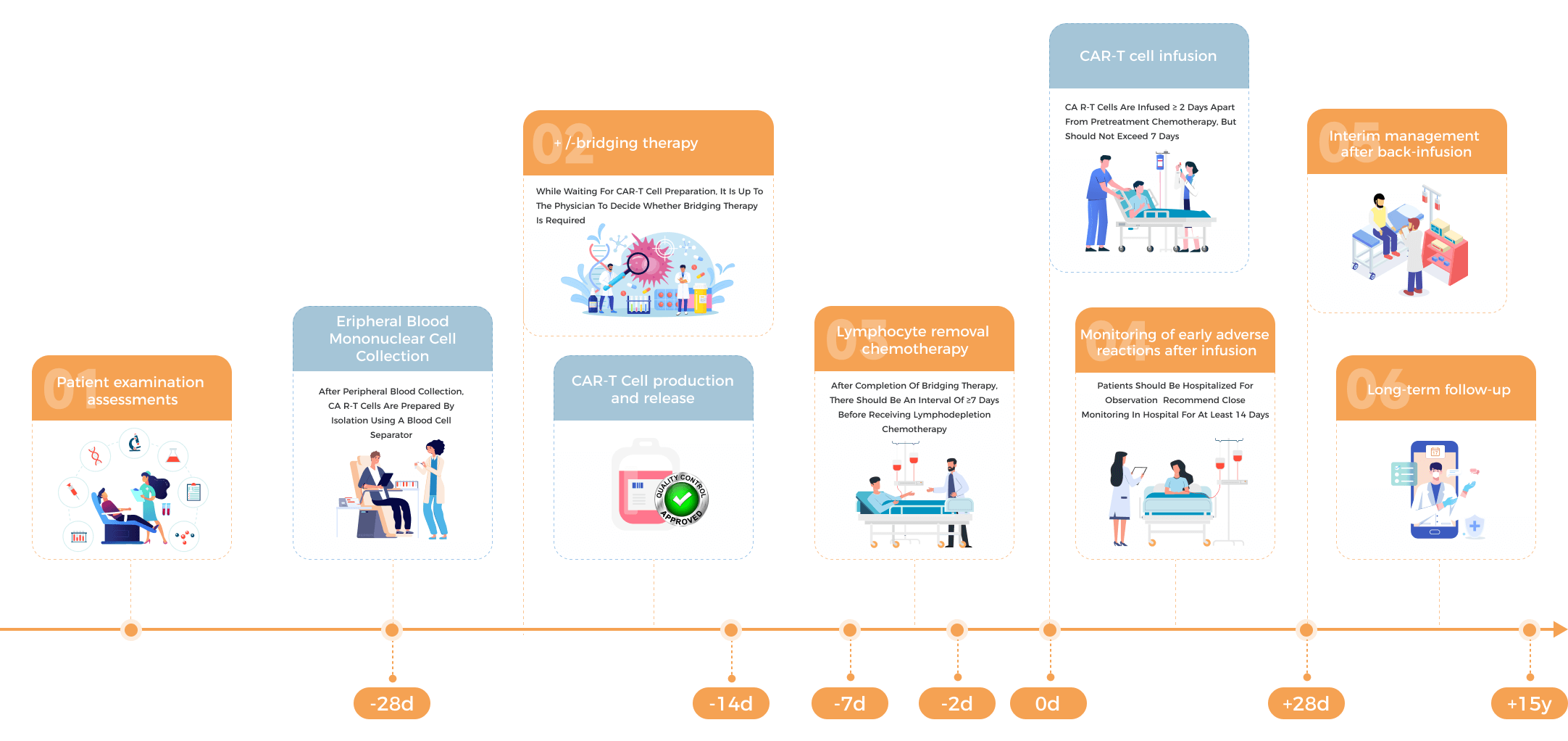
The Simplest Process to Receive Car-T Treatment in China


The unique feature of CAR-T therapy
Single Infusion Only, Short Treatment Cycle
Our Service
Whole Process Planning
- First walk through illness and demand
- Meeting and admission to the hospital
- Security and immigration fraud
- Employment itinerary
- Follow-up to illness
Expert Consultation
- sick collection collection
- sick translation
- Character meeting
- Viewing session
Local Guide Services
- Collaborative clinic contact box
- Qingding Hotel Residence
- Character meeting
- Conveyance machine and transportation
- Daily life assistance
- Daily travel guide and assistance
Hospital Guide Service
- Designated clinic network
- Collaboration support and management, residential hospital reception management
- Clinic translation
- Collaboration development ticket installation
CAR-T Treatment
- CART Cell Technology Service
Family Accommodation
- 2 people/monthly calculation
- Provided accommodation assistance for foreigners (foreigner public security machine planning registration, foreign capital quality hotel, public housing, residential housing selection)
Trusted by Authoritative Media And Foundations
View detailed patient guide View detailed patient guide View detailed patient
Request a Consultation Now for Car-t Programs for Multiple Myeloma
Frequently Asked Questions
How Much Should I Budget for My Car-T Medical Tourism Trip?
When budgeting for a Car-T medical tourism trip, consider various factors including the cost of the treatment itself, travel expenses, accommodation, local transportation, and additional costs for post-treatment care and monitoring. The cost of Car-T therapy can be significant, often ranging from tens of thousands to several hundred thousand dollars, depending on the treatment specifics. Travel and accommodation costs will vary based on the duration of stay, which can be extended due to the need for recovery and follow-up. It's advisable to budget for unforeseen expenses and potential complications. Consulting with our team or the treatment center for an estimated cost breakdown can provide a more accurate budget.
How should I communicate with my doctor about going abroad for Car-T treatment?
Effective communication with your doctor about going abroad for Car-T treatment involves clearly discussing your reasons for seeking treatment overseas, understanding the potential risks and benefits, and gathering all necessary medical information. Share your health history, current treatment details, and any specific concerns. It's crucial to discuss the continuity of care pre and post-treatment. Ask for advice on selecting a reputable treatment center and for any necessary referrals or medical records. Open dialogue ensures that you are well-informed and that your home-country doctor can provide support throughout your treatment journey.
What is the level of Car-T therapy in China?
China has made significant advancements in Car-T therapy, with numerous hospitals and research centers actively engaged in Car-T research and clinical trials. Chinese medical institutions have developed several Car-T products, some of which have been approved for clinical use. These advancements indicate a high level of expertise and commitment to Car-T therapy in China.
Is there a waiting period or queue to receive Car-T for multiple myeloma in China?
In general, there is usually no need for a lengthy waiting period to receive Car-T treatment for multiple myeloma in China. However, the specific timing and scheduling largely depend on the individual medical institutions and their current patient load. For a more precise estimate and to facilitate your treatment journey, it's highly recommended to consult with our experts. We can assist you in identifying the most convenient Car-T treatment centers in China, tailored to your needs. Our team is dedicated to providing you with quick and efficient access to treatment, ensuring that you receive the best possible care without unnecessary delays.
How can I determine if I can go to China for Car-T treatment?
Determining if you can go to China for Car-T treatment involves several steps: Medical Evaluation: Consult with your primary healthcare provider to assess if you are a suitable candidate for Car-T therapy and if traveling is safe for you. Regulatory Considerations: Check visa requirements, medical travel regulations, and any travel advisories for China. Cost and Logistics: Evaluate the financial implications, including treatment costs, travel, accommodation, and any associated expenses. Second Opinion: Consider getting a second opinion from a specialist in Car-T therapy to validate your decision. Plan for Post-Treatment Care: Ensure arrangements are in place for follow-up care upon your return.




